Table 3 shows the San Diego Creek Watershed including the Santa Ana Delhi Channel and other tributaries. The most recent hydrology study for the Santa Ana-Delhi Channel was completed in 1985, entitled AHydrology for Santa Ana-Delhi Channel and Principal Tributaries@ (OCEMA, 1985). Much of the original channel system was designed to convey 65 percent of the 25-year flow rate. Design (ulitmate100-year) flow rates for selected reaches are provided in Table 4.
Lower Newport Bay extends westward about three miles behind the Balboa Peninsula to Newport Boulevard (see Figure 3). The Coast Highway divides the Bay into upper and lower basins. The lower basin is heavily urbanized with numerous islands developed for residential use. The upper basin (about 1,000 acres) remains largely undeveloped within the nominal Bay boundaries with the exception of about the lower one-third, which contains boat docks and other commercial facilities. The remaining area (752 acres) is operated as a State Ecological Reserve by the Department of Fish and Game.
The Upper Bay is characterized by a semidiurnal tidal pattern of two unequal highs and lows occurring each day. The maximum tidal range is about 9 feet (+7.2 ft MLLW to -1.8 ft MLLW), with little difference in absolute magnitude between the upper and lower Bays. Mudflats comprise the lower portion of the littoral zone below about 3.0 MLLW and are subject to daily inundation. Salt marsh occupies the mid and upper littoral zones up to the extreme high water (EHW) elevation. The salinity in the Upper Bay in 1959 was close to seawater (Gerstenberg, undated). The Bay is becoming progressively more estuarine in character as freshwater inputs to the Bay increase.
Water Quality Characteristics
Presented below is a summary of information that is currently available on the water quality characteristics of Upper Newport Bay
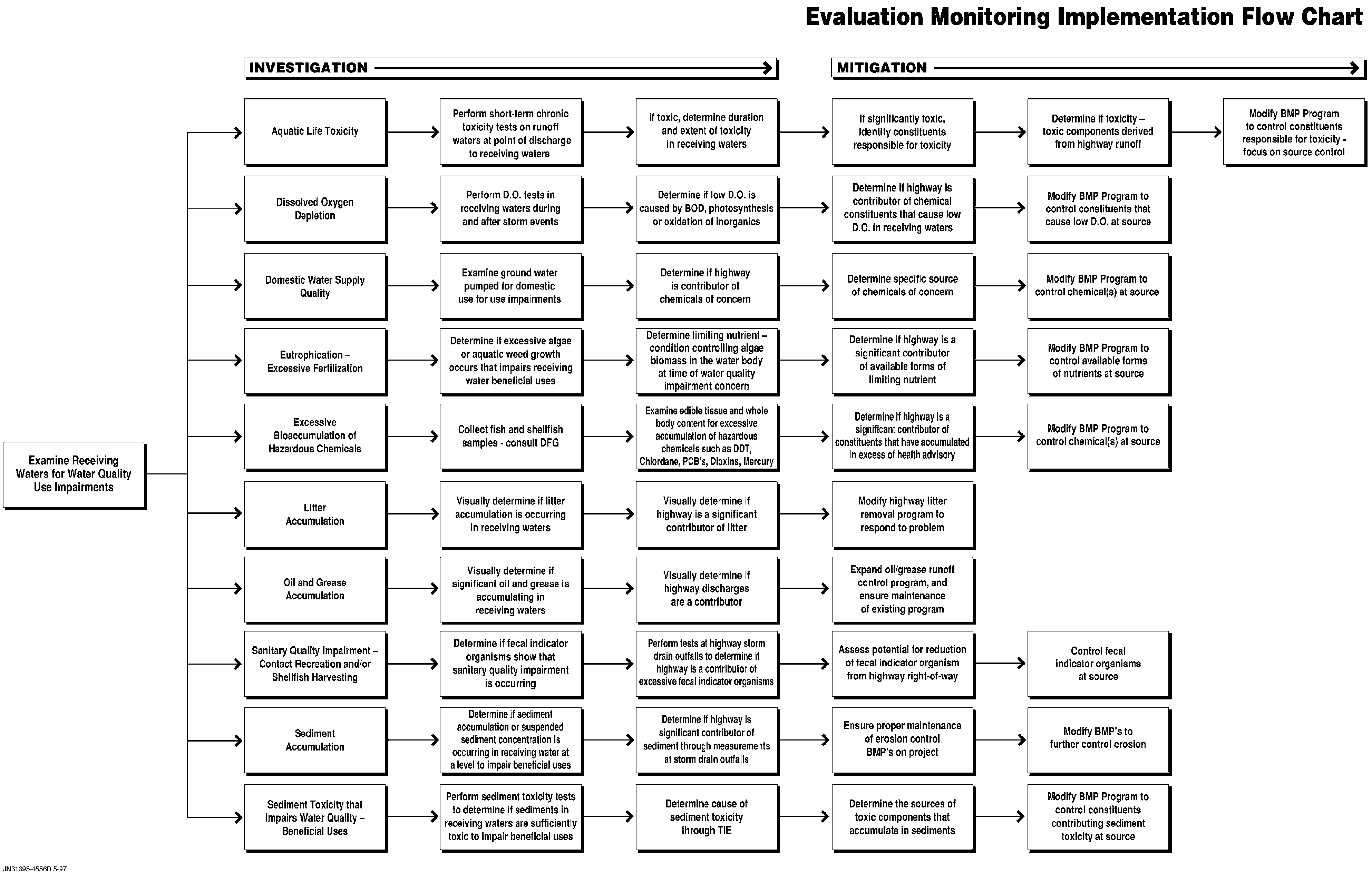
Aquatic Life Toxicity
One area of greatest concern regarding chemical effects on the aquatic life resources of a waterbody is toxicity. Prior to 1970 the primary concern was toxicity that killed adult fish as evidenced when large numbers of dead fish accumulate along the shore. With the advent of effective water pollution control programs in the 1960s, the acute toxicity to adult fish resulting in major fish kills has been largely controlled. Today, while there are exceptions to this situation, they are normally rare.
In the 1970s the emphasis for water pollution control of toxics shifted from acute toxicity to adult fish to acute toxicity to larval fish and fish food such as zooplankton as well as chronic toxicity to larval fish and zooplankton. Both acute and chronic toxicity to small forms of aquatic life are often difficult to detect since there are no readily discernible fish kills. However, the impacts of acute toxicity to small forms of aquatic life that kills the organisms as well as chronic toxicity which, while not killing them, alters reproduction, growth rates, etc., can be important in impairing the aquatic life-related beneficial uses of a waterbody.
Beginning in the late 1960s, the U.S. EPA and its predecessor agencies as well as other agencies began to develop laboratory-based aquatic toxicity tests to determine whether a particular wastewater discharge or stormwater runoff to a waterbody could potentially cause aquatic life toxicity in the waterbody. The first of these tests was the 96 hr LC50 in which fish or zooplankton are placed in a test vessel (a beaker or aquarium) that contains the effluent/runoff water or ambient waters. In a parallel test, the same organisms are exposed to a reference water that does not contain the potentially toxic substances. The number of test organisms that die (lethal concentration) during a 4-day (96-hour) period is determined. While this approach has been developed into a reliable test for acute toxicity, it does not address the issue of chronic toxicity.
Typically the chronic toxicity of chemicals occurs at a factor of 10 to 100 times lower concentrations than acute toxicity (i.e., the acute/chronic ratio is 0.1 to 0.01). For example, if copper is acutely toxic to fish at 100 �g/L, it is likely chronically toxic to fish at about 10 �g/L. In the 1980s, the U.S. EPA and others developed short-term chronic toxicity test procedures in which larval forms of organisms are exposed to potentially toxic waters for a period of several days to a week. These tests typically use larval forms of fathead minnows and a zooplankton, Ceriodaphnia, as test organisms. There is also some testing done with algae, although the algal testing procedures and interpretation of the results involve significantly different approaches than for larval fish and zooplankton. The interpretation and use of planktonic algal toxicity tests in water quality evaluations have been reviewed by Lee and Jones-Lee (1996a). They caution against the strict interpretation of the results of these tests as typically conducted in evaluating a water quality-use impairment for toxicity tests involving fish and zooplankton.
Beginning in the mid-1970s several programs were initiated to determine the presence of toxic chemicals, such as heavy metals and pesticides, in Upper and Lower Newport Bay and its tributaries. According to SARWQCB (1995a), the last time that data of this type were compiled was in 1987. The late-1980 data review that was published by Blodgett (1989) is sufficiently dated so as to not necessarily provide a reliable indication of the present conditions within Upper Newport Bay or its tributaries such as San Diego Creek. From the information available in the late-1980s (associated with the Blodgett review), it appears that a number of the most severe contamination problems associated with Upper and Lower Newport Bay reported in the 1970s and the 1980s have been decreasing, indicating that the water quality programs initiated in the 1980s were effective in beginning to control the potentially toxic chemical constituent input to Newport Bay.
One of the conclusions of the Blodgett (1989) report was that there was need for follow-up studies on the sources and significance of toxic chemicals found in Upper Newport Bay water and organisms. Beginning in the fall of 1992, Olson and Martinez (1993) conducted a study of water quality in Upper and Lower Newport Bays and their tributary channels. This study examined the Upper and Lower Newport Bays' water quality monitoring data over the last 10 years for elevated concentrations of heavy metals and pesticides where a comparison was made between the ambient water concentrations and U.S. EPA water quality criteria. Also, two sets of water and sediment samples (October 1992 and January 1993) were collected and analyzed. One sample set was analyzed for a suite of heavy metals, several organochlorine pesticides, and PCBs. The second set of samples was analyzed for organophosphate pesticides.
It was reported that boatyards located in Lower Newport Bay were apparently a source of high levels of copper present in Bay waters. Further, Olson and Martinez (1993) indicated that there was a potential for copper present in the Lower Bay to be transported to the Upper Bay due to tidal transport. Upper Newport Bay has a 1- to 2-meter tidal cycle, which could account for significant between-bay transport of chemical constituents.
Lead was found at the water quality objective concentrations in the Santa Ana-Delhi Channel runoff waters. Excessive concentrations of heavy metals were primarily located in the Lower Bay. Occasionally, some elevated concentrations of metals were found in Upper Bay and Upper Bay tributary waters.
The five organochlorine pesticides (DDT, endosulfan, dieldrin, endrin, and heptachlor) were present in runoff waters to Upper Newport Bay at concentrations exceeding water quality objectives. Diazinon was present in all four runoff samples tested. PCBs were detected only in sediments in the Lower Bay.
The approach that has been used to date for assessing whether there are toxic substances and potential toxicity to aquatic life in a waterbody has been to measure the concentrations of potentially toxic constituents in the waterbody at various times of the year. The Santa Ana Regional Water Quality Control Board in the Blodgett (1989) report showed that there are a number of chemical constituents such as heavy metals and some organics such as pesticides present in Upper Newport Bay as well as tributary waters at concentrations that are potentially toxic to aquatic life. Olson and Martinez (1993) updated the information on the presence of heavy metals and other potentially toxic constituents in San Diego Creek and Upper Newport Bay waters. They indicated that as of the early 1990s, there were still potentially significant concentrations of some potentially toxic constituents in these waters.
SARWQCB (1995a) stated that there are excessive trace metals and organics in San Diego Creek and at certain locations in the Bay. Urban stormwater runoff is specifically mentioned a source of potentially toxic substances. SARWQCB states that additional efforts should focus on more specific identification of toxic compounds.
Recently, as part of the development of the IRWD's Wetlands Water Supply Project, CH2M HILL (1996) has reported on the aquatic life resources of San Diego Creek just upstream of Upper Newport Bay. In this region, it was found (MBC, 1995) that San Diego Creek has a number of freshwater fish, including carp, chubs, and shiners. Evidently, these fish are reproducing in San Diego Creek or its tributaries. This indicates that San Diego Creek is not highly toxic to fish.
Bailey et al. (1993) conducted toxicity tests on San Diego Creek and several other tributaries of Upper Newport Bay. These tests used larval fathead minnow, fish; Ceriodaphnia dubia, zooplankton; and Selenastrum capriocornutum, alga. These samples were collected between November 1992 and January 1993. All samples were collected within 12 to 24 hours of a rainfall event and processed at the University of California Davis Aquatic Toxicology Laboratory. The sampling stations included San Diego Creek at Campus Drive, Santa Ana-Delhi Channel at Irvine Avenue, San Diego Creek at Culver Drive, and Peters Canyon Channel at Barranca Highway. Peters Canyon Channel is a tributary of San Diego Creek. Santa Ana-Delhi Channel is a major stormwater runoff tributary of Upper Newport Bay that primarily drains urban and industrial/commercial areas.
Except for one sample from the Santa Ana-Delhi Channel, all samples showed no significant toxicity compared to the controls for the fathead minnows. However, the tests conducted with Ceriodaphnia for San Diego Creek (Campus Drive), the Santa Ana-Delhi Channel, and San Diego Creek (Culver Drive) all caused complete mortality of the test organism. The sample from Peters Canyon Channel showed no toxicity based on death of the organism in the first set of samples. However, in the second set of samples, there was 100 percent mortality at this location. The testing with the alga Selenastrum showed no inhibition of algal growth, which indicates the waters did not contain constituents at the time of sampling that would inhibit algal growth.
As part of the Olson and Martinez (1993) studies, chemical testing of stormwater runoff found diazinon, a pesticide, and diuron, a herbicide, at potentially significant concentrations. The work did not confirm whether the toxicity found was due to these chemicals or if these chemicals were present in sufficient concentrations to be potentially toxic.
Diuron is a herbicide that is used on highway right of ways. However, it is unknown whether it was being used in the Upper Newport Bay Watershed on highway right of ways at the time of the studies in late 1992 and early 1993. In 1990 over 1,300 pounds of diuron were applied to right of ways in Orange County. Also, over 1,200 pounds were applied in the county for structural pest control (Department of Pesticide Regulation [DPR], 1994). Powell and Leyva (1994) found toxic concentrations of the herbicide diuron associated with its use on highway right of ways. These studies were not conducted in the Orange County area.
Previous studies conclude that there were toxicants to zooplankton - Ceriodaphnia in stormwater runoff in several of the major tributaries of Upper Newport Bay in 1992-93. However, the chemical(s) responsible for this toxicity was not identified.
Studies conducted by the Central Valley Regional Water Quality Control Board staff and the USGS Sacramento Office as well as others (Domagaiski, 1995; Kuivila, 1993; Kuivila and Foe, 1995; Connor, 1995; Foe, 1995a,b; USGS, 1993; Cooper, 1996; Hansen & Associates, 1995; Katznelson and Mumley et. al., 1997; Waller et. al., 1995; Bailey et. al., 1996; McConnell et. al., 1997) have found that stormwater runoff from urban and rural areas contains organophosphate pesticides that are acutely toxic to Ceriodaphnia. Because Ceriodaphnia toxicity was previously found in San Diego Creek waters by Bailey et. al. (1993), and because diazinon has been reported to be present in San Diego Creek waters (Olsen and Martinez,(1993), there is need to determine whether the previously reported toxicity to this organism is still occurring today in tributaries of Upper Newport Bay. If toxicity is found in Upper Newport Bay tributary waters, then studies are needed to determine its cause and significance in potentially adversely affecting the beneficial uses of Upper Newport Bay as well as San Diego Creek. This issue has been addressed as part of the Phase I field monitoring activities presented in the report by Lee and Taylor (1997).
The traditional approach for assessing a potential aquatic life toxicity in a waterbody is to measure the concentrations of a suite of potentially toxic chemicals for which the U.S. EPA has developed water quality criteria. The previous reviews, Blodgett (1989), Olson and Martinez (1993), and the SARWQCB (1995a), have all reported excessive concentrations of some heavy metals and other constituents in Upper Newport Bay and San Diego Creek waters relative to U.S. EPA water quality criteria. Considerable additional data has been collected by OCEMA now Orange County Public Facilities and Resources Department in their stormwater monitoring of San Diego Creek and other tributaries of Upper Newport Bay as well as Bay waters since the last comprehensive review of the data on the chemical concentrations of potentially toxic constituents such as heavy metals was conducted in the early 1990s by Olson and Martinez. It is, therefore, appropriate to review the more recent monitoring data on tributary and Bay waters to determine if there are potentially excessive concentrations of heavy metals and other constituents that could, if they are in toxic-available forms for a sufficient period of time, cause aquatic life toxicity to San Diego Creek and/or Upper Newport Bay aquatic life.
OCPFRD is a co-permittee with the 31 municipalities in Orange County for the NPDES stormwater permit covering urban area stormwater runoff water quality management. OCEMA has been conducting a stormwater runoff monitoring program as part of this permit since May 1991. OCPFRD operates a network of monitoring stations located throughout the county, which includes San Diego Creek and its tributaries as well as at various locations within Upper Newport Bay. Two reports have been issued by OCEMA that present the data obtained in their stormwater runoff monitoring. OCEMA (1994) presents the data for the period 1991 through the spring of 1994. The data collected after this time through the spring of 1996 is presented in OCEMA (1996).
OCPFRD's monitoring includes measurement of flow, various physical parameters, electrical conductivity, turbidity, pH, nitrate, ammonia, total kjeldahl nitrogen, phosphate, total suspended solids, volatile suspended solids, cadmium, chromium, copper, lead, nickel, silver, zinc, and hardness in the water column. Some of the heavy metal measurements in the water column included filtering the sample before analysis to determine the dissolved metals. Also, determinations have been made of the same heavy metals, DDD, DDE, DDT, gamma BHC, 2,4-D, 2,4,5-TP, silvex, benzo(k) fluoroanthene, and pyrene in the Newport Bay and the Santa Ana River watershed sediments. The primary thrust of the OCPFRD stormwater runoff monitoring program is the assessment of the concentrations of potentially toxic constituents.
A review of this database shows that the tributaries of Upper Newport Bay contain sufficient concentrations of nitrate, copper, and zinc to potentially be adverse to water quality in the tributaries as well as within Upper Newport Bay. Upper Newport Bay, near where San Diego Creek enters it, was found to contain total copper, lead, nickel, silver, and zinc concentrations above U.S. EPA water quality criteria. DDT and its breakdown products DDD and DDE were found in Upper Newport Bay tributary waters and within the Bay at concentrations that could lead to excessive bioaccumulation within aquatic life. Polyaromatic hydrocarbons (benzo(k) fluoroanthene and pyrene) and several heavy metals were found in Newport Bay sediments at elevated concentrations.
The stormwater runoff monitoring data currently available focuses on determining selected chemical constituent concentrations in runoff waters and to some extent in receiving waters and their sediments. While a number of the chemical constituents are found at concentrations above U.S. EPA water quality criteria or former state water quality objectives, that in general are numerically equal to the U.S. EPA criteria, this exceedance of the regulatory objectives cannot be used to determine whether real water quality-use impairments are occurring.
The constituents present at elevated concentrations relative to water quality criteria/objectives are likely present to some and possibly substantial extent in non-toxic, non-available forms, with the result that while there could be an "administrative" exceedance of a water quality criterion/objective, this exceedance may not be reflective of real water quality problems that would impair the use of the tributaries or the Bay waters by the public. For example, many of the heavy metals monitored are of concern because of their potential to cause aquatic life toxicity. However, it is not possible to relate the concentration of total or dissolved metals as measured in the available data to actual aquatic life toxicity in the waterbody in which the metals are present. It is for this reason that ambient water toxicity measurements of the type described in the Evaluation Monitoring Program developed by Silverado (1997) focus on measurement of aquatic life toxicity to directly assess whether measured as well as unmeasured constituents are responsible for potential water quality-use impairments in the tributaries to Upper Newport Bay as well as in the Bay waters. This issue is discussed further by Lee and Taylor (1997).
Currently sediment monitoring data has provided information on the total concentrations of a variety of metals and other constituents in Upper Newport Bay and other waterbody sediments. The data, however, cannot be used to assess whether constituents in the sediments are in toxic/available forms and whether the measured as well as the unmeasured constituents in the sediments are adversely impacting the beneficial uses of the waterbody in which the sediments are located. Again, as with water column measurements, it is not possible to determine from chemical concentrations of constituents in sediments whether elevated concentrations of heavy metals, polyaromatic hydrocarbons (PAHs), chlorinated hydrocarbon pesticides, etc., are in toxic/available forms that could be adverse to the numbers, types, and characteristics of desirable forms of aquatic life in the waterbodies in which the sediments are located. This issue has been reviewed by Silverado (1997) as part of developing the Evaluation Monitoring Program and this Demonstration Project.
Elevated concentrations of potentially toxic constituents, such as heavy metals, in a tributary's stormwater runoff waters do not necessarily translate to excessive concentrations in the receiving waters for the runoff. There are a wide variety of physical, chemical, and biological factors that must be evaluated to determine whether the constituents in the runoff waters are toxic to the aquatic life. Significantly elevated concentrations of a variety of the constituents of concern can be present in stormwater runoff from urban areas and highways without adversely impacting the beneficial uses of the waterbody.
As discussed by Lee and Jones-Lee (1997a), increasing recognition is being given to the potential significance of chromium VI as a toxicant to certain forms of aquatic life, especially zooplankton. The Environment Canada (1995) reporting of the toxicity of chromium VI to some forms of zooplankton (Ceriodaphnia reticulata) at 0.5 �g/L raises concern about the impact of urban area stormwater runoff as a source of chromium that can be toxic to some forms of aquatic life. Mount (1997) has indicated that the toxicity of chromium VI to Ceriodaphnia dubia occurs at less than the U.S. EPA water quality criterion of 10 �g/L. Pitt and Field (1990), as reported by Lee and Jones (1991a), found that the median concentration of total chromium in urban stormwater runoff obtained during the U.S. EPA National Urban Runoff Program (NURP) studies was 30 �g/L.
The mean concentration of soluble total chromium in stormwater runoff from urban area streets across the U.S. was 1.3 �g/L. While no information is available on whether this chromium was in a chromium III or VI form, since it was soluble and since chromium III tends to strongly sorb to particulates, it is possible that chromium VI occurs in urban area stormwater runoff at a concentration that could be toxic to some Ceriodaphnia and other zooplankton species. It is also possible that some of the toxicity that is now attributed to organophosphate pesticides in urban area stormwater runoff that is particularly significant to Ceriodaphnia could be due to chromium VI. This is an area that needs further attention.
While there have been extensive measurements of the concentrations of a variety of potentially toxic chemical constituents in Upper Newport Bay tributaries and Bay waters, these studies have not yielded definitive information as to whether water quality-use impairments have in the past or are now occurring due to toxic chemicals. There is need to conduct further studies to determine whether San Diego Creek waters entering Upper Newport Bay are toxic to aquatic life. If toxicity in the Creek waters is found, then an evaluation should be conducted on the significance of this toxicity to aquatic life within San Diego Creek and Upper Newport Bay. If toxicity that is judged to be significant with respect to potentially impairing the beneficial uses of Upper Newport Bay and/or San Diego Creek waters is found, then the cause of this toxicity and the sources of the constituents that cause the toxicity should be identified and, if possible, controlled at the source.
Bioaccumulation of Hazardous Chemicals
The bioaccumulation of hazardous chemicals in aquatic organism tissue is of concern since some of the bioaccumulated chemicals occur at sufficient concentrations in fish tissue to increase the risk of cancer or other adverse impacts to those who eat the fish. This has lead the Food and Drug Administration (FDA) to develop action levels to determine when fish contain excessive concentrations of hazardous chemicals in their edible tissue. The FDA action levels, however, incorporate a number of factors including economic considerations that tend, for some chemicals that bioaccumulate in fish tissue, to represent an increased risk of adverse impacts over that typically considered to be acceptable today. Several years ago it was somewhat arbitrarily determined that an acceptable increased cancer risk of one additional cancer in a million people exposed to the hazardous chemical for their lifetime was an "acceptable" risk. This value is commonly used today for determining whether drinking waters, aquatic organisms used as food, etc., contain sufficient concentrations of a hazardous chemical or groups of chemicals to represent a significant threat to public health.
The water quality criteria listed in the U.S. EPA (1987) "Gold Book" for bioaccumulatable chemicals were designed to prevent excessive bioaccumulation of hazardous chemicals that represent either an exceedance of FDA action levels in fish tissue or an increased cancer risk of one cancer in a million people who consume 6.5 g of fish per day (one meal per month) taken from the waters that contain the hazardous chemical of concern. More recently, the U.S. EPA has focused its effort for the control of hazardous chemicals that tend to bioaccumulate in fish on a risk-based approach (an increase in cancer risk). This is the approach that is widely used today for the chlorinated hydrocarbon pesticides (DDT, chlordane, etc.), PCBs, and dioxins. A similar approach is being used to establish water quality criteria for mercury. Mercury tends to bioaccumulate in fish tissue to levels that represent potential neurological damage to those who consume fish with elevated concentrations of mercury in their tissue. Recently, the U.S. EPA (1993a, 1994, 1995a) has released a series of guidance manuals devoted to bioaccumulation issues that serve as the background to the Agency's current approach for determination of excessive concentrations of hazardous chemicals in fish tissue. These manuals should be consulted for further background information on this matter.
One issue of concern regarding evaluating bioaccumulation data for the hazardous chemical content of fish and other aquatic life is the limited reliable information available to determine an excessive concentration of hazardous chemicals in fish with respect to the use of fish and other organisms food. However, it is now well-established that certain high molecular weight chlorinated hydrocarbons, such as DDT and PCBs, that tend to bioaccumulate in fish can cause reproductive impairment for fish-eating birds.
In estimating the critical concentrations of chlorinated hydrocarbons and certain heavy metals, such as mercury, in fish tissue that represent a hazard for human consumption, estimates have to be made regarding the amount of fish containing the hazardous chemicals that are consumed on a routine basis. Traditionally, the U.S. EPA has assumed that the average fish consumption rate in the U.S. is 6.5 g of fish per person per day (one meal per month). While that value may be appropriate for the average consumption, it does not protect those who eat local fish as a major component of their diet. In the San Francisco Bay region, the U.S. EPA has suggested that the average consumption rate of fish for some people in that region should be increased to 30 g per person per day (i.e., one meal per week).
In the discussions that follow devoted to the interpretation of the existing bioaccumulation data for fish from Upper Newport Bay and its tributaries, both fish consumption rates are considered. As discussed below, there are a number of chemicals where fish from Upper Newport Bay and its tributaries have tissue concentrations of hazardous chemicals of concern in the range between the values that result from the 30 g of fish per day and the 6.5 g of fish per day per person.
One issue that needs to be addressed for Upper Newport Bay water quality evaluation is to what extent people in the region eat fish from Upper Newport Bay tributaries. From the characteristics of the tributaries, such as San Diego Creek and its tributaries, it could be that there is no consumption of tributary water fish by humans, and, therefore, the human health aspects of bioaccumulation issues for Upper Newport Bay focus exclusively on fish from Upper Newport Bay and Newport Bay. However, wildlife that eat fish from San Diego Creek and its tributaries must be considered.
Some wildlife-based water quality criteria have been developed in the Great Lakes region (U.S. EPA, 1993b) to address excessive concentrations of hazardous chemicals in fish and other aquatic life that represent a threat to higher trophic level organisms that eat fish. However, there are questions as to whether the Great Lakes-based water quality criteria are appropriate for use in other areas. The Great Lakes have somewhat unique water quality characteristics compared to many waterbodies that, in general, would cause chemicals in the Great Lakes at particular concentrations in the waters to be more of a threat to the beneficial uses of a waterbody than occurs in most other waterbodies. This would be especially true in attempting to translate wildlife water quality criteria developed for the Great Lakes to Upper Newport Bay. Much higher concentrations of a potentially bioaccumulatable chemical can likely be present in Upper Newport Bay waters compared to Great Lakes waters without causing excessive bioaccumulation since there are a number of constituents in Upper Newport Bay waters that would tend to bind/reduce the availability of potentially bioaccumulatable chemicals compared to what commonly occurs in the Great Lakes.
The San Francisco Regional Water Quality Control Board, as part of the Bay Protection Toxic Cleanup Program data collection of the WRCB, has conducted a survey of the concentrations of various hazardous chemicals in San Francisco Bay fish. This survey resulted in a report presenting these results (SFRWQCB, 1995). To assess excessive concentrations of chlorinated hydrocarbons and mercury in fish tissue, the SFRWQCB has used several sets of tissue concentration screening values. One of these is the U.S. EPA guidance value based on a 30 g fish per person per day consumption rate. The other is based on the traditional 6.5 g of fish consumed per person per day. SFRWQCB also provided information on FDA action levels. These three screening levels are used in this study to examine whether fish taken from San Diego Creek and Upper Newport Bay contain concentrations of hazardous chemicals in their tissue to represent a potential public health threat to those who use the organisms as food. Except for PCBs, it is not possible to examine whether there are excessive concentrations of hazardous chemicals in fish that would represent a threat to wildlife since, at this time, the only wildlife-based criterion available is the U.S. EPA's Great Lakes' value for total PCBs.
The SFRWQCB (1995) also presented information on screening values based on so-called National Academy of Science (NAS) values as well as Maximum Tissue Residue Levels (MTRL) values. The "NAS" values have been used in California in an attempt to interpret data obtained in the state's Toxic Substances Monitoring (TSM) program. Several years ago the State Water Resources Control Board staff adopted the NAS values as a guide to determine excessive concentrations of chemicals in aquatic life tissue. These values were numeric concentrations that were suggested by the National Academy of Science and the National Academy of Engineering (NAE) in their 1972 Blue Book of water quality criteria. The chairman of the NAS/NAE (1973) Blue Book Criteria Committee (Fetterolf, 1996) who was also former chief biologist for the state of Michigan water pollution control program and former director of the Great Lakes Fisheries Commission, has indicated that it is inappropriate to use the 1972 "NAS" Blue Book values as being reliable today for estimating excessive concentrations of chemicals in aquatic life tissue. The U.S. EPA, any other state, and the National Academy of Science do not recognize the "NAS" values used by the SWRCB as reliable screening values for determining excessive concentrations of chemicals in aquatic organism tissue.
The MTRL values used by the SWRCB are also not reliable values for estimating concentrations of constituents in aquatic life tissue. These values are based on an extrapolation from water quality criteria and a standard bioaccumulation factor to estimate concentrations within fish tissue that would be adverse to the use of fish used as food. This approach, however, with few exceptions, overestimates the bioaccumulation that will occur because the bioaccumulation of a chemical depends upon the available forms of the constituent in the water column. The concentration of available forms is dependent upon site-specific characteristics with particular reference to those constituents that tend to cause bioaccumulatable chemicals to be unavailable for bioaccumulation. Applying standard bioaccumulation factors to Upper Newport Bay fish would be inappropriate since, in general, less bioaccumulation would be expected in Upper Newport Bay and its tributaries than occurs in many other waterbodies.
A number of the constituents measured in sediments as part of the OCPFRD stormwater runoff monitoring program, such as DDT, are of concern because of their potential to bioaccumulate in aquatic life to excessive levels, causing the aquatic life tissue to be hazardous for use as human food due to an increased cancer or public health risk. However, it is neither possible from either water column nor sediment concentration data to reliably predict whether the constituents reported in this data are in forms that can bioaccumulate within aquatic life tissues to excessive levels. As discussed elsewhere in this report, excessive bioaccumulation of some constituents in San Diego Creek and Upper Newport Bay aquatic life has been found in the past by the Water Resources Control Board's Toxic Substances Monitoring Program. Therefore, at least some of the potentially bioaccumulatable chemicals present in San Diego Creek and Upper Newport Bay waters/sediments are in available forms. The data, however, does not provide the information necessary to evaluate whether the potentially bioaccumulatable constituents measured at a particular location in the water column or sediments are responsible for the excessive bioaccumulation found in the past and is likely occurring today. To develop information necessary to determine whether the concentrations of potentially bioaccumulatable constituents in the water and/or sediments are present in forms that are significantly contributing to the bioaccumulation problem, if it still exists today, it is necessary to conduct special purpose studies like those described in the Evaluation Monitoring Program section of the ETC Runoff Management Plan (Silverado 1997) as well as Lee and Jones-Lee (1997b).
One type of chemicals of increasing concern because of excessive bioaccumulation in aquatic life tissue is dioxins. Dioxins are formed from combustion processes, including automobile exhaust, and they are present in vehicular traffic exhaust. They would, therefore, be expected to be ubiquitous in the environment. Recently the San Francisco Regional Water Quality Control Board released a report entitled, "A Survey of Stormwater Runoff for Dioxins in the San Francisco Bay Area" (SFRWQCB, 1997), which provides data on the dioxin content of urban area street and highway stormwater runoff. The concentrations of dioxins being found are a factor of 10 to 100 above U.S. EPA-proposed drinking water standards. While no information is provided on the water quality significance of the dioxins in the stormwater runoff, the fact that dioxins are being found at excessive levels in fish taken from various waterbodies, such as San Francisco Bay, is of concern. The San Francisco Regional Water Quality Control Board (SFRWQCB, 1995) has found that fish in San Francisco Bay have excessive levels of dioxins compared to U.S. EPA guidelines for protection of public health associated with consumption of fish containing dioxins.
Since dioxins tend to be strongly sorbed to particulates, it is likely that they are present in stormwater runoff in a particulate form. They are likely associated with small particulates and would not be effectively removed in detention basins. Further work needs to be done to evaluate this situation. No information is available at this time on the relative contributions of highway and street stormwater runoff as a source of dioxins compared to other sources. Ultimately, it will be appropriate for future studies beyond this Demonstration program to examine fish from San Diego Creek and Upper Newport Bay for excessive concentrations of dioxins to determine whether this is a potential problem in the Creek and Bay waters.
A constituent of potential concern in highway and urban area street runoff is lead. Lead concentrations in stormwater runoff frequently exceed U.S. EPA water quality criteria. Further, the concentrations of lead in soils and sediments near highways are often greatly elevated compared to soils from other areas. Lee and Jones-Lee (1992, 1997c) have reviewed the public health aspects of lead in soil and water. They point out that lead in both of these media tends to be over-regulated compared to current understanding of the impacts of lead on aquatic life and human health. One of the areas of particular concern is the potential for lead to bioaccumulate in aquatic life to a sufficient extent to represent a threat to children's health who eat fish with elevated concentrations of lead.
The Agency for Toxic Substances and Disease Registry (Cox, 1997) has, as part of a study of bioaccumulation of potentially hazardous chemicals in Putah Creek fish and other aquatic life near the University of California, Davis Department of Energy Laboratory for Energy-Related Health Research (LEHR) National Superfund site, developed a guidance value for lead in fish tissue. This value is based on a comparison between the consumption of drinking water at the U.S. EPA action level of 15 �g/L, assuming 2 L/day consumption, and the consumption of fish, assuming a 50 g/day consumption rate. The 50 g/day consumption rate is somewhat above the values typically used by the U.S. EPA of 6.5 g/day and 30 g/day, i.e. one meal per month or one meal per week, respectively. It is on the order of two meals per week. It is important to understand that the 15 �g/L lead drinking water action level is not necessarily a safe concentration. The U.S. EPA in developing that level recommended that the concentration be kept as low as possible. The 15 �g/L level should be protective of children in order to keep their blood lead levels below 10 �g/dL. The 10 �g/dL blood level is a value that is often used to indicate excessive blood lead that is possibly harmful to a child. There is some evidence, however, that blood lead levels below this concentration are adverse to children. It is concluded that any time the concentrations of lead in fish tissue used for human food are above about 0.3 mg/kg, there should be concern for children making extensive use of these fish as food.
Lee and Jones-Lee (1996b) developed a review of the current understanding of the relationships between the concentrations of chemical constituents in the water column or sediments and excessive bioaccumulation in aquatic life tissue. They have pointed out that it is not possible to predict the extent of bioaccumulation that will occur in fish tissue based on chemical concentrations in the water column and/or sediments. Site-specific evaluation of water column and/or sediment associated constituents have to be made in order to determine whether a constituent in either area is or could become a source of excessive bioaccumulation.
Chlorinated Hydrocarbons Beginning in the 1970s, the State Water Resources Control Board initiated a TSM program that included determination of the concentrations of the various potentially hazardous chemicals in aquatic life taken from the waters of the state (WRCB, 1995). The TSM has included sampling San Diego Creek and its tributaries as well as Upper Newport Bay fish. Presented below is a discussion of the TSM data for fish taken from San Diego Creek and Upper Newport Bay for the chemicals that have been analyzed as part of the WRCB TSM. Generally, the data reported for the hazardous chemical tissue concentration in fish is based on a composite of six fish taken within a certain area at one time.
Total Chlordane Chlordane is a chlorinated hydrocarbon pesticide that has been extensively used for termite control and other purposes. Its use is no longer allowed. Based on the approach used for evaluating excessive bioaccumulation of organic chemicals in San Francisco Bay (SFWRQCB, 1995), the critical concentration of total chlordane in fish for those who consume 30 g of fish per day, i.e., one meal per week, for a 70-kg adult is about 18 �g/kg. The corresponding value for consuming 6.5 g fish per day, i.e., one meal per month, for a 70-kg adult is 80 �g/kg. The WRCB's TSM database shows that fish, such as red shiner, taken from San Diego Creek at various locations as well as some of its tributaries such as Peters Canyon Channel, contain total chlordane of above the 18 �g/kg wet weight risk-base level that is considered hazardous to human consumption. Three sets of samples were taken of Newport Bay marine fish in 1990-1992. The total chlordane content of these fish was less than 18 �g/kg.
It may be concluded from the database available that fish in the tributaries of Upper Newport Bay contain excessive chlordane compared to a risk-based projected cancer risk level for humans who consume on the order of 30 g of fish per day taken from these waters. However, there are questions about whether there are any significant fisheries - human consumption of fish taken from the tributaries of Upper Newport Bay.
It would be appropriate to do additional sampling of fish from Upper Newport Bay to determine whether the three sets of samples taken in the early 1990s are representative of today's conditions. Further, additional sampling of fish in San Diego Creek and several of its tributaries should be conducted to confirm that the excessive levels of chlordane found primarily in the mid-to-late 1980s to early 1990s is still occurring today.
Aldrin Aldrin is a chlorinated hydrocarbon pesticide that is banned for further use because of its persistence and potential to be a health hazard to increase the cancer risk to those exposed to it. Sampling of San Diego Creek as well as Upper Newport Bay over the years has not found excessive concentrations of aldrin in fish tissue compared to the FDA action level of 0.3 mg/kg wet weight. The U.S. EPA has not established a guideline value for aldrin in fish tissue.
Total DDT DDT is a chlorinated hydrocarbon pesticide that was used for many years for a variety of pest control purposes. Its use was banned because of its persistence and its potential to cause cancer in those exposed to it. The critical level for total DDT based on the 30 g/day of fish consumption is 68.6 �g/kg. For a 6.5 g per day fish consumption rate, the critical concentration is 300 �g/kg. The San Diego Creek fish samples, with few exceptions, had total DDT concentrations in excess of both values. The three sets of fish collected from Upper Newport Bay in the early 1990s had total DDT concentrations below the 300 �g/kg hazard level, but two of the three sets of fish had DDT above the 68.6 level. The DDT level in the one set of fish was below the 68.6 �g/kg level.
The FDA action level for DDT is 5,000 �g/kg wet weight. Therefore, the fish in Upper Newport Bay as well as in San Diego Creek do not exceed the FDA action level for DDT. They are, however, considered hazardous based on a U.S. EPA risk-based screening level that is designed to protect humans from the one in a million increased cancer risk. There is need for additional sampling to determine whether the situation today is still the same as previously found with respect to total DDT in fish taken from Upper Newport Bay and its tributaries.
Dieldrin Dieldrin is a chlorinated hydrocarbon pesticide that had been banned because of its potential to bioaccumulate and cause cancer. The critical levels for dieldrin for 30 g per day fish consumption are 1.5 �g/kg and for 6.5 g per day is 7.0 �g/kg. The FDA action level for dieldrin is 300 �g/kg. Many of the fish taken from San Diego Creek and its tributaries had excessive concentrations of dieldrin compared to screening values based on both fish consumption rates. The detection limit that was used in these studies was 5 �g/kg. Therefore, it cannot be determined whether all the fish taken from San Diego Creek have excessive levels of dieldrin. All fish taken from Upper Newport Bay had less than the 5 �g/kg detection limit used for dieldrin analyses. There is need for additional sampling for dieldrin, using a more sensitive analytical approach to be sure that concentrations measured are at less than 1 �g/kg wet weight.
Total Endosulfan Total endosulfan is a chlorinated hydrocarbon pesticide that was banned because of its potential to bioaccumulate and cause cancer. The total endosulfan hazard level for consumption of 30 g of fish per day is 3,500 �g/kg. For the 6.5 g of fish per day consumption rate, it is 20,000 �g/kg. The concentrations of total endosulfan of fish taken from San Diego Creek and its tributaries as well as from Upper Newport Bay were well below these levels.
Heptachlor Epoxide Heptachlor epoxide is a chlorinated hydrocarbon pesticide that was banned because of its potential to bioaccumulate and cause cancer. The critical concentration for 30 g of fish per day is 2.6 �g/kg and for 6.5 g of fish per day is 10 �g/kg. The FDA action level is 300 �g/kg for heptachlor epoxide. Several samples of fish were taken from San Diego Creek that had concentrations of heptachlor epoxide between 5 and 16 �g/kg. Most of the samples taken from San Diego Creek and those taken from Upper Newport Bay had concentrations of heptachlor epoxide below the detection level of 5 �g/kg. The analytical methods used, however, do not detect heptachlor epoxide with sufficient sensitivity to determine whether excessive concentrations were present in the fish from Upper Newport Bay and San Diego Creek.
PCBs PCBs (arochlors) are chlorinated hydrocarbons (non-pesticides) that have been primarily used as an electrical transformer fluid as well as for a variety of other purposes. The excessive concentration for total arochlors (PCBs) for 30 g of fish per day consumption is 3 �g/kg and for 6.5 g of fish per day is 10 �g/kg. The FDA action level for total PCBs is 2,000 �g/kg. The total PCB content of fish taken from San Diego Creek and its tributaries was well in excess of both the 30 and 6.5 g of fish per day consumption rate. The fish were only taken from Upper Newport Bay and analyzed for PCBs in 1990 and 1991. No analyses were conducted in 1992. The 1990-1991 samples contained total PCBs at about 100 �g/kg, well above the critical level. It, therefore, may be concluded that PCBs are present in Upper Newport Bay tributaries and in the Bay fish at excessive concentrations that represent a public health hazard to those who eat the fish.
The U.S. EPA Great Lakes wildlife criterion for total PCBs is 0.2 to 1 �g/kg. Therefore, the San Diego Creek and Upper Newport Bay fish have sufficient concentrations of total PCBs to be potentially adverse to wildlife that eat the fish.
Toxaphene Toxaphene is a chlorinated hydrocarbon pesticide that was banned because of its potential to bioaccumulate and cause cancer. The critical concentrations for toxaphene are 21 �g/kg for 30 g of fish per day and 300 �g/kg for 6.5 g of fish per day. The concentrations of total toxaphene in fish taken from San Diego Creek and its tributaries were in some cases well above the critical concentration. The detection limit used for measuring toxaphene in fish from Upper Newport Bay was 100 �g/kg, and, therefore, there could be excessive toxaphene in Upper Newport Bay fish that was not detected by the monitoring program analytical methods used.
Oxadizon While there are no critical concentrations for oxadizon listed, the concentrations found in fish in some samples taken from Upper Newport Bay were in excess of 2,200 �g/kg. According to Rasmussen (1996), oxadizon is a herbicide that the manufacturer claims does not bioaccumulate in fish. However, it is accumulating in large amounts in Upper Newport Bay fish tissue. The hazard of this accumulation is unknown.
Overall Assessment It can be concluded that fish in San Diego Creek and several of its tributaries contain excessive concentrations for several formerly used chlorinated hydrocarbon pesticides, such as chlordane, DDT, and dieldrin. These fish also contain excessive concentrations of PCBs relative to those that are considered of increased cancer risk to humans who eat these fish as frequently as one meal per month.
The Toxic Substances Monitoring Program of the Water Resources Control Board does not include analysis for dioxins. It would be of interest to determine if excessive concentrations of dioxins are found in San Diego Creek and Upper Newport Bay fish.
It is unclear whether the excessive concentrations of chlorinated hydrocarbon pesticides and PCBs in San Diego Creek fish represent a human health hazard, even though the concentrations found within the fish tissue exceed human health advisory levels for the use of the fish as food. There is need to better understand whether there is any fishing in these tributaries to assess whether there is a human health hazard.
Based on studies in other places, there is likely to be a wildlife hazard due to excessive concentrations of some of the pesticides and PCBs in these fish for those animals and especially birds that eat the fish. PCBs and DDT are well-known to cause reproductive failure in fish-eating birds. This could be a problem associated with a bird population that consumes fish from San Diego Creek and its tributaries.
There is need for additional sampling of San Diego Creek and especially Upper Newport Bay to determine whether the various chlorinated hydrocarbon pesticides and PCBs, which in the early 1980s through the early 1990s were present in excessive concentrations in fish tissue, are still present in excessive concentrations today. These chemicals have not been used for a number of years, and, therefore, in many parts of the country their concentrations in fish are decreasing. Further, since other areas are finding excessive concentrations of dioxin in fish, dioxin should be measured in the San Diego Creek, and especially Upper Newport Bay fish.
Heavy Metals Based on the WRCB TSM data, the heavy metal content of San Diego Creek and its tributary fish as well as Newport Bay fish is generally below critical levels, except for an occasional exceedance of the mercury level of 0.14 �g/kg for the 30 g of fish per day consumption rate. Therefore, bioaccumulation of mercury in fish is a potential problem in both San Diego Creek tributaries and Upper Newport Bay. A similar situation exists with lead compared to the value that was developed by the Agency for Toxic Substances and Disease Registry (ATSDR) for the Putah Creek fish. There are some values of lead concentrations in fish tissue from Upper Newport Bay tributaries and the Bay that exceed the guideline value of 0.3 mg/kg.
The lead exceedances are not considered significant problems, however, since the number of exceedances compared to total number of samples is quite low. It would be appropriate to take an additional set of samples from Upper Newport Bay as well as at a couple of locations on San Diego Creek to confirm that the situation today with respect to mercury and lead in fish is still, as it has been in the past, at a low level of contamination and not a significant threat to human consumption of fish taken from these areas.
Mussel Watch For the period 1978 through 1995, mussels were suspended in cages at various locations in Upper Newport Bay. This was done as part of the WRCB's mussel watch program, where mussels are used to assess whether excessive concentrations of potentially hazardous chemicals accumulate within mussel tissue (WRCB, 1996a). Periodically, the mussels are removed from their cages and their tissue is analyzed for a number of chemical constituents of potential concern.
The total chlordane concentrations in the mussel samples taken from Upper Newport Bay typically ranged between the 18 �g/kg concentration value and 80 �g/kg. Similarly, the total DDT content of the mussels exposed in Upper Newport Bay ranged from less than the 68.6 �g/kg guideline value for 30 g of fish per day to less than the 300 �g/kg value for 6.5 g of fish consumed per day. Many mussels had concentrations between these two values. Similarly, the total PCB content of mussels suspended in Upper Newport Bay waters is typically above the 10 �g/kg guideline value for 6.5 g of fish per day consumption rate. Some samples accumulated total PCBs in excess of 400 �g/kg.
Some of the mussels accumulated cadmium in excess of the 2.2 mg/kg and 10 mg/kg guidance values. Many of the mussels accumulated cadmium between the two guideline values. A similar pattern was found for mercury accumulation in mussels. Many mussels accumulated mercury in excess of the 0.14 mg/kg guideline value for 30 g of shellfish consumption per day; there were only a few, however, that accumulated in excess of 0.6 mg/kg. While most of the mussels had lead concentrations below the ATSDR recommended limit, there were some mussels with concentrations of lead at or near that limit. Several heavy metals that are analyzed as part of the TSM do not have critical concentrations tissue values, such as chromium, copper, manganese, nickel, titanium, zinc, silver, aluminum, and arsenic. It is, therefore, not possible to judge whether the concentrations found are excessive. However, normally these metals do not represent significant threats to those who eat fish and other aquatic life.
Care must be exercised in using mussel watch data to interpret the actual bioaccumulation of hazardous chemicals that is occurring in edible organisms in a region, especially if they are near the critical concentration levels for excessive bioaccumulation. As discussed by Salazar et al. (1995), minor changes in some of the variables associated with mussel watch such as organism size, etc., within the range that is normally allowed, can significantly impact the bioaccumulation of constituents in mussel tissue. The exposure conditions that occur in mussel watch sampling can be significantly different from the exposure conditions that ambient water organisms experience in a region. It is, therefore, important that any regulatory activities devoted to bioaccumulation issues be focused on actual organisms that are used as food and not be based on mussel watch data, especially if the data is near the critical concentration value for a chemical of concern.
Need for Additional Studies There is need for additional monitoring of fish from Upper Newport Bay and San Diego Creek to establish the current levels of fish tissue contamination relative to those that are potentially hazardous for use of the fish as food. There is also need to better understand whether there is any significant use of San Diego Creek fish as human food. Because of the limited water available in San Diego Creek during much of the year and the limited size of the fish normally present in the Creek, it is likely that there is limited human health threat associated with consuming fish from the creek even if they still contain excessive concentrations of chlorinated hydrocarbon pesticides, PCBs, and mercury. There may, however, be a potential wildlife threat, especially to fish-eating birds due to the excessive concentrations of the chlorinated hydrocarbon pesticides and PCBs that could be present in San Diego Creek fish.
Aquatic life in some areas, especially associated with petroleum hydrocarbon refining and industrial processes that introduce large amounts of PAHs into a waterbody, has been found to have tumors, lesions, and other illnesses associated with the chemicals that are carcinogens. While this is apparently not a problem associated with urban area and highway stormwater runoff, it would be important to examine some of the aquatic organisms in an area receiving such runoff to determine if they have tumors, liver or other organ lesions, abnormal organs, etc., that could be attributable to the constituents in the runoff.
At this time, no information is available on whether aquatic life within Upper Newport Bay and its tributaries are experiencing disease due to pathogenic organisms as well as carcinogens and other chemicals that cause tumors and/or other abnormal tissue growth within the organism. Also, no information is available as to whether there are any endocrine or other hormone-active substances that impair Upper Newport Bay aquatic organism and water fowl reproduction/behavior. Crisp, et al. (1997) have recently issued a report on behalf of the U.S. EPA that concludes that insufficient information is available to determine whether there are hormone-disrupting chemicals in the environment that are adversely affecting public health and the environment. This is an emerging area of increasing national/international water quality concern that could be significant to Upper Newport Bay. It is likely, however, based on what is known from other areas, since generally, where these problems have been found, they appear to be associated with major industrial wastewater inputs. Problems of this type may be associated with past and possibly current pesticide/herbicide inputs to the Bay. The current pesticide/herbicide registration process used by the U.S. EPA does not properly evaluate the potential for these types of chemicals to be adverse to aquatic life and wildlife.
Sediments as a Source of Bioaccumulatable Chemicals
Since many chemicals of concern with respect to excessive concentrations in aquatic organism tissue are chemicals that are no longer being used, such as the chlorinated hydrocarbon pesticides and PCBs, it appears that the aquatic sediments and possibly the soils within the Upper Newport Bay watershed and within the Bay are the source of these chemicals that is maintaining the elevated concentrations within the aquatic life tissue. If additional studies show that aquatic life within San Diego Creek and Upper Newport Bay still contain excessive concentrations of many of the constituents of concern because of their human health threat, studies will need to be conducted to determine the specific sources of the constituents responsible for the excessive bioaccumulation. However, it is inappropriate to assume that the concentrations of constituents in sediments that tend to bioaccumulate are a reliable estimate of the significance of a sediment as a hazardous chemical source that accumulates to excessive levels.
As discussed by Lee and Jones-Lee (1996b), there are a wide variety of factors not related to the concentration of a hazardous chemical in sediments that control the availability of such chemicals for bioaccumulation. These are bulk chemical characteristics of the sediments that include total organic carbon (TOC), carbonate, hydrous metal oxide, and sulfide content, which control the ability of a constituent in sediments to be a source of constituents that bioaccumulate in aquatic organism tissue. Accordingly, low concentrations of a potentially hazardous constituent can readily bioaccumulate to higher levels within aquatic life tissue than high concentrations of the same constituent in a different sediment that has greater sediment binding for the constituent than the sediments that have lower concentrations.
The approach that must be used to address this issue is site-specific evaluations using aquatic organism exposure under controlled conditions to determine if a constituent in sediments is available for bioaccumulation. There are a variety of factors such as the opportunity for higher trophic organisms to be exposed to conditions that lead to bioaccumulation that influence whether available forms of constituents in sediments that must be evaluated on a site-specific basis to determine whether a chemical in sediments is, in fact, the source of the constituents that are bioaccumulating to excessive levels in the fish of a region.
It is possible that chemical constituents in urban area and highway stormwater runoff could accumulate in receiving water sediments causing a significant use impairment of these waters through sediment toxicity, and/or serving as a source of chemicals that leads to excessive bioaccumulation of hazardous chemicals in aquatic organism tissue. The accumulation of chemicals in sediments can be due to either particulate forms of the constituent in the stormwater runoff, or dissolved forms in the runoff that become particulate in the receiving waters through sorption, precipitation, and/or bio-uptake by lower trophic level organisms, such as algae, which die, settle, and become part of the sediments.
It is not possible to use total chemical concentrations in sediments to reliably predict water quality problems associated with heavy metals, organics, and other chemical constituents. It became evident in the 1970s through the U.S. Army Corps of Engineers (COE) Dredged Material Research Program results that it is necessary to use biological effects-based evaluations of potential water quality impacts (toxicity and bioaccumulation) to determine if heavy metals, or other constituents in sediments, are significantly impairing the beneficial uses of a waterbody. Biological effects-based techniques are well established to determine whether potentially toxic constituents that accumulate in sediments are adverse to the waterbody. Since the mid-1970s, the U.S. EPA and COE have been regulating excessive concentrations of chemicals in sediments associated with navigational waterway dredging and dredged sediment disposal as they may impact the beneficial uses of the waterbody in which the disposal takes place through the use of biological effects-based approaches such as toxicity tests and bioaccumulation. In 1991, the U.S. EPA and the COE updated their Testing Manual for ocean disposal of contaminated sediments (U.S. EPA/COE, 1991). The Agency and Corps are now updating their freshwater dredged sediment disposal manual based on similar approaches to those used for nearly 20 years (U.S. EPA/COE, 1994). A discussion of the development and use of these procedures is provided by Lee and Jones (1992) and Lee and Jones-Lee (1994a) as well as Wright (1992).
Lee and Jones-Lee (1993a; 1996c,d) have reviewed issues pertinent to evaluating the water quality significance of chemical constituents in aquatic sediments. As they discuss, both aquatic life toxicity to a suite of sensitive aquatic organisms, and bioaccumulation in aquatic organism tissue of chemicals that are of potential concern to human health and wildlife should be evaluated as part of a biological effects-based sediment quality assessment. Selected chemical analyses, coupled with toxicity tests of the sediment, should be made as part of the TIE evaluation conducted to determine the cause of the toxicity for the regulated chemicals such heavy metals, PAHs, and ammonia, and for the constituents in aquatic sediments that tend to detoxify/immobilize chemicals (TOC, sulfides, etc.). Toxicity tests, field bioaccumulation studies, and benthic aquatic organism assemblages (numbers, types and characteristics) should be used in a non-numeric, best professional judgment, weight-of-evidence triad to determine whether aquatic sediments in the vicinity and downstream of a stormwater runoff point significantly contribute to the impairment of the designated beneficial uses of the receiving waters for the runoff.
It is possible to conduct a sediment-based TIE to determine the cause of the toxicity for those sediments found to have sufficient toxicity to impair the beneficial uses of the waterbody. Ankley et al. (1991) are developing guidance on conducting TIEs on aquatic sediments. The information developed from the TIE can then be used to develop a technically valid, cost-effective approach for implementing stormwater runoff BMPs.
It is important to not use chemically-based approaches for estimating toxicity such as Long and Morgan values, McDonald values, and apparent effect threshold (AET) values for assessing water quality impacts of sediment-associated constituents. Such co-occurrence-based approaches are unreliable (Lee and Jones-Lee, 1996c) because they are based on total concentrations of constituents in sediments and a contrived co-occurrence with a non-cause-and-effect biological characteristic of the sediments. Sediment water quality impacts should be based on biological effects-based assessments with appropriately used chemical information associated with a TIE (Lee and Jones-Lee, 1993a, 1996d).
Finding toxicity in aquatic sediments should not be interpreted to mean that this toxicity is a significant cause of a beneficial use impairment for the waterbody in which the sediments are located. As discussed by Lee and Jones-Lee (1996d), many aquatic sediments are naturally toxic due primarily to the growth of algae and other aquatic plants in the waterbody, which, upon death, accumulate in sediments and exert an oxygen demand that consumes the dissolved oxygen (DO) in the sediments. The slow rate of diffusion of oxygen into sediments from the overlying waters and the high inorganic oxygen demand typically present in sediments leads to no DO in the sediments below the superficial layer. Low DO conditions in sediments lead to the accumulation of ammonia and hydrogen sulfide, both of which are highly toxic to aquatic life. While these conditions occur naturally, the activities of man in a waterbody's watershed can increase the amounts of aquatic plant nutrients contributed to a waterbody and therefore the toxicity of the sediments.
The natural toxicity of sediments due to low DO, NH3, and H2S is not necessarily a significant factor in adversely affecting the designated beneficial uses of waterbodies. Many waterbodies with toxic sediments have desirable aquatic life resources. At this time, there is a poor understanding of the coupling between sediment toxicity and the impairment of the designated beneficial uses of waterbodies. Work needs to be done to understand how the control of constituents in stormwater runoff, which accumulate in receiving water sediments (causing or contributing to sediment toxicity), influences the beneficial uses of waterbodies.
There have been a number of studies of Upper Newport Bay and Newport Bay sediment chemical characteristics. Olson and Martinez (1993) reported that the heavy metal concentrations in Upper Newport Bay sediments were not found to be significantly elevated from what would be expected and would not likely represent a significant cause of sediment toxicity. As discussed by Lee and Jones (1992) and Lee and Jones-Lee (1993a), it is not possible to relate the concentration of a constituent in sediments to water quality impacts. The concentration of a constituent in sediments depends not only on the concentration of the constituent of concern, which is related to its rate of deposition into the sediments, but also on the rates of deposition of the bulk sediment components such as sand, clays, silts, organic matter, carbonates, etc. In a situation such as Upper Newport Bay where there are large amounts of erosional material added to the Bay sediments, it is possible that the erosional material does not detoxify toxic forms of constituents in sediments. It does, however, reduce their concentration. These concentrations could still be sufficient to be toxic to aquatic life even though they are not significantly elevated from the expected concentrations for sediments of this type. It is, therefore, necessary to directly measure the toxicity of sediments to judge whether a constituent in the sediments is, in fact, toxic.
Olson and Martinez (1993) found a correlation between DDT concentration in sediments and the total organic carbon concentration in Upper Newport Bay sediments. This is to be expected since the incorporation of organic chemicals such as DDT in aquatic sediments is primarily through sorption on organic carbon particles.
It was also reported by Olson and Martinez (1993) that the boatyards located in Lower Newport Bay were apparently a source of high copper levels present in Newport Bay waters and sediments. Further, Olson and Martinez indicate that there is a potential for copper present in the Lower Bay to be transported by the tides to the Upper Bay. Upper Newport Bay has a 1- to 2-meter tidal cycle, which could account for significant between-bay transport of chemical constituents.
As part of the Bay Protection and Toxic Cleanup Program (BPTCP) designation of toxic hotspots in the state's enclosed bays and coastal waters, the SARWQCB (1995) has designated Upper Newport Bay as a toxic hotspot based on the presence of elevated concentrations of several heavy metals and pesticides in Bay waters, sediments, and/or aquatic life. The constituents of concern included lead, copper, cadmium, and several pesticides, including dacthal, DDT, endosulfan, chlordane, chlorpyrifos, diazinon, lindane, heptachlorepoxide, and polychlorinated biphenyl (PCB). (Note: PCBs are not pesticides but are chlorinated hydrocarbons that have similar adverse impacts as the chlorinated hydrocarbon pesticides.) The toxic hotspot designation is based on the presence of elevated concentrations of the chemical and does not consider whether the chemical is in a toxic/available form that could adversely impact the designated beneficial uses of the waterbody. One or more of these chemicals could be present in Upper Newport Bay sediments in non-toxic forms.
Recently, a set of sediment characteristic data has been obtained on Upper Newport Bay as part of the U.S. EPA's EMAP/Water Resources Control Board's BPTCP (WRCB, 1994; 1996b). A total of 18 stations, 5 of which were located in Upper Newport Bay, were sampled once in 1994 for the purpose of determining sediment toxicity and selected chemical characteristics. The chemicals measured included a suite of heavy metals, chlorinated hydrocarbon pesticides, PCBs, and PAHs. Also, total and un-ionized ammonia was measured as well as total organic carbon. No measurements were made of total sulfide content of sediments, with the result that an important sediment characteristic, with respect to determining whether the heavy metals in the sediments are a potential cause of toxicity, was not included in the BPTCP study program. This is a significant omission since it is known that the sulfide content of the sediments is an important characteristic for assessing the role of heavy metals as a cause of sediment toxicity.
Two amphipods, Rhepoxynius abronius (RA) Ampelisca abdita (AA), and Strongylocentrotus purpuratus (SPPF) (sea urchin eggs) were used as test species in the sediment toxicity testing. While the BPTCP EMAP program used a number of other test organisms such as other amphipods, abalone larvae, mussel larvae, and polychaetes in toxicity testing in other locations, these organisms were not used in the Upper Newport Bay toxicity testing. This is unfortunate in that, as discussed by Lee and Jones-Lee (1996d), to properly assess the toxicity of sediments, a suite of sensitive organisms should be used. Further, from the BPTCP data collected in 1994 on Upper Newport Bay sediments, it is not possible to evaluate information that was collected with respect to the reference sediment testing that was done. The BPTCP data printout, where this information should have been included, indicated that no statistics were developed for comparing the toxicity of the test samples to the "home" sediments used. Possibly when the BPTCP report is developed for the 1994 data, information of this type will be available in that report.
In the data interpretation presented below, it is assumed that the toxicity data reported was statistically significantly different from the toxicity found in appropriately conducted reference sediment tests. If this subsequently proves to be inappropriate, then it can be concluded, because of the low levels of toxicity found to the amphipods tested, that, in general, the Upper Newport Bay sediments do not have statistically significant toxicity for the organisms tested.
Another potential problem with the BPTCP sediment toxicity evaluations for Upper Newport Bay is that the sea urchin fertilization test was used. This test is known (Lee, 1991) to yield a significant number of false positives where apparent toxicity is found that is not due to definable chemical characteristics of the test water. Factors other than anthropogenically derived chemical constituents, as well as natural pollutants, appear to cause toxic responses in the sea urchin fertilization test.
This review focuses on the Upper Newport Bay sediment characteristics data that were developed for the sampling locations north of the Coast Highway. This approach was followed since it was believed that the constituent impacts in San Diego Creek and Upper Newport Bay's other major tributaries, including the Santa Ana Delhi Channel, would be primarily manifested in this part of the Upper Newport Bay - Newport Bay area. Newport Bay is the part of this waterbody that is south of the Coast Highway. That area is apparently affected by local inputs of constituents derived from boat mooring areas and boatworks. While there is the possibility of materials moving upgradient from Newport Bay to Upper Newport Bay via tidal action, it appears from the data available that the primary source of constituents for Upper Newport Bay sediments is San Diego Creek drainage with some contribution from the Santa Ana Delhi Channel and stormwater runoff from Newport Beach, Santa Ana Heights, and Costa Mesa that directly enters Upper Newport Bay through local storm sewers.
The uppermost sediment sampling point (85018 D, see Figure 3) was located in Unit Basin 1 in the San Diego Creek channel. Apparently, this sampling location represents sediments that have accumulated in a San Diego Creek sediment accumulation basin designed to trap sediments eroded from the San Diego Creek watershed as they enter Upper Newport Bay. The toxicity testing of this sediment with the two amphipods showed that there was a small amount of apparent toxicity as manifested by 89 � 11 percent survival for RA and 86 �13 percent survival for AA. High levels of toxicity were found with the sea urchin development and fertilization tests. This sample contained about 0.2 mg/L NH3 un-ionized ammonia.
At the station just below the former Salt Works (85017 R) located in the main channel of Upper Newport Bay downstream of Jamboree Road where San Diego Creek enters Upper Newport Bay, the amphipod tests showed limited toxicity with RA at 93 � 6 percent survival and AA with 87 � 13 percent survival. Again, as with the sample of the Unit Basin 1 sediments, the sea urchin fertilization test and embryo development test showed high levels of toxicity. The un-ionized ammonia at this location was measured as 0.2 mg/L NH3.
The Upper Newport Bay sampling location (85017 D) just below the former barrier dam in the region of the former ski zone basin also showed low levels of toxicity to the two amphipods with RA showing 81 � 4 percent survival and AA at 93 � 6 percent survival. The sea urchin development test showed high levels of toxicity while the fertilization test showed no toxicity. The un-ionized ammonia at this location was about 0.1 mg/L NH3.
Sampling station 85001 R was located in the main channel to the west of Upper Island just below the narrows. Of the amphipods, only RA was tested with a 29 � 15 percent survival rate. The sea urchin development test showed high levels of toxicity while there were moderate levels of toxicity in the sea urchin fertilization test. Un-ionized ammonia at this location was 0.6 mg/L NH3.
Sampling station 85008 R was located in the main channel of Upper Newport Bay just downstream of Shellmaker Island and south of the two peninsulas. The RA survival was 57 � 14 percent; AA was not tested. The test with the sea urchin fertilization and development showed high levels of toxicity. High levels of un-ionized ammonia, 1.6 mg/L NH3, were present in the sediments at this station.
Sampling station 85009 R was located in Upper Newport Bay to the north of the Newport Dunes Resort and south of Polaris Drive. Low levels of toxicity were found for both amphipods with RA showing 93 � 6 percent survival and AA showing 57 � 10 percent survival. Again, high levels of toxicity to sea urchin egg fertilization and development were found. Un-ionized ammonia was 0.3 mg/L NH3.
It can be concluded that Upper Newport Bay sediments near where San Diego Creek enters the Bay show low levels of toxicity to the amphipods tested. However, the two stations in the mid-part of the Upper Bay near Upper Island and Shellmaker Island both showed elevated levels of toxicity to the organisms tested. The levels of un-ionized ammonia were sufficient at these stations to be toxic to some forms of benthic organisms. As discussed by Lee and Jones-Lee (1996d), a number of important benthic and epibenthic organisms, such as shellfish larvae, are killed by un-ionized ammonia at a few tenths of a mg/L NH3. Therefore, even if a sample does not show toxicity to amphipods, some of which tend to have less sensitivity to ammonia toxicity than mussel and fish larvae (Pinza et al., 1996), it is possible that the ammonia accumulating in sediments as a result of organic nitrogen derived from dead algae and other sources could be adverse to aquatic life.
The two stations where elevated toxicity to RA was found were stations that apparently have fine-grained sediments. RA is well known to be sensitive to fine-grained sediments that cause an apparent toxicity. For this reason, RA is not a particularly suitable test organism for fine-grained sediments.
Review of the total concentrations of heavy metal data (arsenic, antimony, chromium, copper, cadmium, lead, mercury, nickel, silver, and zinc) obtained at the various sampling stations where toxicity was tested shows that these sediments contain the heavy metals, with the exception of zinc, for three stations at or below the mean concentrations of these metals in California soils as reported by Dragun and Chiasson (1991). For zinc, the concentrations found in the Upper Newport Bay sediment samples just downstream of Jamboree Road as well as in the lower part of the Bay just above the Coast Highway were somewhat elevated compared to normal California soils. The concentrations of zinc found in these sediments, however, would not be expected to be toxic, since the concentrations are typical of those found in many sediments for which no aquatic life toxicity is found.
It may be concluded that Upper Newport Bay sediments do not contain heavy metals at concentrations that would be expected to be responsible for the small amounts of aquatic life toxicity found for the amphipods tested. Since many of the sediments contained potentially significant concentrations of ammonia, it is likely that they also contain elevated concentrations of total sulfide. Therefore, even if the metals had been elevated compared to normal soil concentrations, it is possible that the sulfides would have detoxified the metals through precipitation reactions.
It is not possible to interpret the sea urchin toxicity data since, as noted above, data of this type is not necessarily a reliable indication of an adverse impact to aquatic life within the sediments or to the beneficial uses of a waterbody.
Overall, it does not appear that Upper Newport Bay stormwater runoff is contributing heavy metals and other constituents to the Bay that are accumulating in sediments leading to potentially significant aquatic life toxicity. While there is some aquatic life toxicity, the levels for amphipods are not particularly high. The sediments, as expected, contain elevated concentrations of ammonia and likely contain elevated concentrations of sulfides. These are derived primarily from the input of nitrogen and phosphorus that stimulate excessive algal growth. The algae, upon dying, settle to the bottom and result in the depletion of the dissolved oxygen in the sediments due to bacterial respiration of the dead algal cells. While this is a source of sediment toxicity, it is possible that it is not a significant factor in Upper Newport Bay beneficial uses since many eutrophic waterbodies that have similar types of sediments have outstanding fishery resources.
An important consideration when interpreting the chemical concentration data for constituents that tend to accumulate in sediments is the flux of total sediment to the system. The high rates of erosion and the filling of Upper Newport Bay with erosional sediments would significantly dilute elevated concentrations of constituents derived from stormwater runoff from urban areas and highways. Therefore, even though there is substantial urban area and highway stormwater runoff entering Upper Newport Bay and with it associated heavy metals and other constituents, the large amounts of erosional materials from the watershed tend to dilute the urban area and highway stormwater runoff constituents to lower levels than would be found in areas where urban area and highway stormwater runoff is the principal source of sediment in the waterbody. An example of this type of situation occurs at some of the sampling stations of Newport Bay sediments where significantly elevated concentrations of copper apparently derived from local boatworks are found. The areas where the copper has accumulated are not areas that are apparently experiencing rapid filling due to watershed erosion.
It may be surmised from the limited data available that Upper Newport Bay sediments do not, at this time, represent a potentially significant cause of aquatic life toxicity that would adversely impair the designated beneficial uses of Upper Newport Bay. Therefore, it is proposed that sediment toxicity is an area that should be a low priority for Evaluation Monitoring funding. If further testing is done, it should be conducted with a suite of test organisms whose toxicity response has potential relevance to the impairment of the designated beneficial uses of Upper Newport Bay. If further testing shows toxicity to a number of potentially ecologically significant organisms, then sediment TIE work should be done to determine the constituents responsible for this toxicity. Once they have been defined, then forensic studies involving a combination of chemical and toxicity testing should be used to determine the sources of the constituents responsible for the sediment toxicity. It will be necessary to evaluate the relative significance of anthropogenically derived constituents responsible for sediment toxicity versus the toxicity associated with the excessive fertilization of the Bay due to low DO and elevated H2S and ammonia.
Eutrophication/Excessive Fertilization
The excessive fertilization of waterbodies is one of the major causes of water quality-use impairment. This impairment affects the aesthetic quality of waters used for recreational purposes with the appearance of excessive algal and waterweed growth. For domestic water supplies, excessive fertilization leads to a number of problems, such as increased taste and odors, shortened filter runs, and at some locations, increased trihalomethane precursors. As discussed by Jones and Lee (1982, 1986) and Lee and Jones (1991b), while increasing the fertility of a waterbody results in an overall increased fish biomass, increased fertility generally results in a deteriorated quality of fish where less desirable, rough fish, such as carp, become predominant. Lee and Jones (1991a) and Lee and Jones-Lee (1996e) have discussed the importance of evaluating the potential significance of urban area and highway stormwater runoff-derived nutrient loads compared to other sources of nutrients for a waterbody. Rast and Lee (1984) have provided guidance on how this can be accomplished.
Per unit area, urban area streets tend to export more nitrogen and phosphorus per year than most agricultural/rural lands. An important exception occurs with dairies and some other animal husbandry activities. There are situations where urban street runoff has caused excessive fertilization of small urban lakes (Lee and Jones, 1980). Ordinarily, however, excessively fertile waterbodies near urban areas and highways obtain most of their nutrients from domestic wastewater sources, agricultural and rural land runoff, the atmosphere, or from nitrogen compounds in groundwater that discharge to the waterbodies.
Several important issues need to be addressed in developing nutrient-based BMPs for stormwater runoff. One of these is the need to focus the nutrient control program on those forms of nutrients (N and P) that can stimulate algal growth in the receiving waters. For most freshwater systems, the nutrient control program must be focused on algal-available phosphorus and not total phosphorus. Similarly, for those waterbodies in which nitrogen is the chemical controlling algal biomass that develops in the waterbody, the control programs must focus on available forms of nitrogen compounds. For marine waters, it is typically the algal available nitrogen that is the key constituent in controlling algal biomass in the receiving waters, although there may be situations where phosphorus can become an important element in controlling algal growth in estuarine and nearshore marine waters. A site-specific evaluation of the relative significance of nitrogen versus phosphorus in controlling excessive fertilization of a waterbody must be made to determine whether algal-available forms of the controlling element present in stormwater runoff and other nutrient sources are significant contributors to the excessive fertility of the waterbody.
Lee et al. (1980) have provided guidance on the determination of available forms of aquatic plant nutrients in runoff waters and sediments. Basically, for nitrogen it is the nitrate plus ammonia plus part of the organic nitrogen in the runoff waters that become available in the receiving waters to support algal growth. For phosphorus, it is the sum of the soluble orthophosphate plus about 20 percent of the particulate phosphorus that is available to support algal growth in stormwater runoff. Site-specific determinations of available N and P can be assessed through the use of algal bioassays.
Lee and Jones (1988a,b) provided guidance on the approaches that can be used to determine whether nitrogen or phosphorus is the key limiting element in controlling algal biomass in a waterbody. As they point out, a number of the approaches that are used, such as the ratios of N and P in the waterbody, are not necessarily reliable and can lead to incorrect conclusions on the significance of nitrogen or phosphorus in controlling algal growth. In making an assessment of the limiting nutrient, it is important to ascertain whether the proposed limiting nutrient is present at algal growth rate limiting concentrations during peak algal biomass. If it is found that during the peak of the algal bloom the algae still have available to them surplus amounts of available forms of nitrogen and phosphorus, then these elements are not limiting the algal biomass.
It is also important to consider the hydraulic/morphologic characteristics of the waterbody (flushing time) receiving the stormwater runoff at various times of the year. If it is found that the nutrients added to the waterbody during one time of the year are effectively flushed out of the waterbody before the period of the year when excessive algal growth occurs, then the nutrients contributed to the waters during the non-growth periods are not contributing to the eutrophication-related water quality problems.
Further, a distinction should be made between: (1) eutrophication-related water quality problems, which are manifested as excessive growths of planktonic algae, and (2) the growths of attached algae, attached and floating macrophytes, and emergent vegetation. With respect to the latter, there is a poor understanding of nutrient load-concentrations/eutrophication response relationships.
Jones and Lee (1982, 1986) have provided guidance on how to evaluate the potential benefits of controlling phosphorus inputs to a certain degree on the eutrophication-related water quality of a waterbody. Lee and Jones (1986) have found that at least a 25 percent reduction in the total available phosphorus load to the waterbody during the period of water quality concern must occur before a discernible improvement in the planktonic algal-related water quality will occur. At this time, similar relationships have not been developed for nitrogen. However, it is likely that at least the same magnitude of control of algal available nitrogen must occur before there will be a discernible improvement in eutrophication-related water quality of a waterbody due to nitrogen input control.
The Blodgett (1989) report addressed the algal bloom problems of Upper Newport Bay. The algal blooms in Upper Newport Bay were attributed to nutrient, especially nitrate, input from nurseries and other agricultural practices. Studies conducted between 1985 and 1988 showed mean concentrations of nitrate in Upper Bay waters ranging from 1 mg/L to as much as 80 mg/L as NO3-. High levels of dissolved oxygen (greater than 5 mg/L) were observed in Upper Newport Bay throughout the year indicating that, at least to the extent that the sampling for DO was representative, excessive fertilization of the Bay was not leading to low DO problems. That situation may not be occurring today since recent studies by Irvine Ranch Water District indicate that low DO may be related to excessive fertilization of the Bay.
SARWQCB (1995) discusses the eutrophication problems of Upper Newport Bay where it indicates that seasonal algal blooms occur, creating a recreational and aesthetic nuisance. Algal blooms may also adversely affect wildlife through aquatic life toxicity and altering habitat. The excessive fertilization of Upper Newport Bay has been attributed to nitrate input to the Bay. Gerstenberg (undated), in a report on the Management Plan for the Upper Newport Bay Ecological Reserve, has summarized the information available on the excessive fertilization of Upper Newport Bay. He indicates that previous investigators (MBC, 1980) have found that nitrate concentrations in Upper Newport Bay waters decreased to growth-rate-limiting concentrations during summer algal blooms.
It appears that nitrate is the key element controlling excessive fertilization of Upper Newport Bay. This nitrate is primarily derived from commercial nurseries and agricultural sources, although there were significant amounts of nitrate derived from urban runoff (Smythe, 1990). The specific sources of the urban area runoff nitrate were not determined. A source of nitrate that has not been adequately characterized is the high nitrate in the surficial shallow groundwaters that enters San Diego Creek and its tributaries. Some of these groundwaters have been found to contain nitrate N at concentrations of 50 mg/L or greater. Since this groundwater discharges continuously to some of the tributaries of Upper Newport Bay, it could be an important source of nitrate for the Bay especially during the summer months when there is no stormwater runoff. Further, there could be direct discharge of nitrate in groundwaters to the Bay through subsurface Bay discharges.
As part of the development of the Evaluation Monitoring Program for the Eastern Transportation Corridor, the author (Dr. Lee) (Silverado, 1997) has reviewed the information available on Upper Newport Bay eutrophication water quality problems as well as the recent OCEMA nutrient data for San Diego Creek and Upper Newport Bay obtained as part of the OCEMA stormwater runoff monitoring program (OCEMA 1994, 1996). Further, the recently collected data by the Irvine Ranch Water District (IRWD) as part of the monitoring program that is being conducted associated with the Wetlands Water Supply Project has been examined (IRWD, 1996). Also, the author of this report has personally examined the Bay at various times during 1995 and 1996 to review the excessive fertilization that is occurring in the Bay waters. Based on the published reports, data review, and personal observations, the following conclusions can be developed with respect to excessive fertilization of Upper Newport Bay.
Excessive fertilization is significantly impairing the beneficial uses of Upper Newport Bay. It is manifested primarily as attached and floating algal growth, which begins to occur in excessive amounts in mid-spring and lasts through the summer and early fall. At this time, it appears that nitrogen compounds (nitrate, nitrite, and ammonia, as well as organic nitrogen that converts to ammonia/nitrate) are the key chemicals potentially limiting further algal growth within the Bay. The concentrations of available forms of phosphorus in Bay waters, at times, may become sufficiently low to limit algal growth in the Bay.
The information available indicates, however, that for much of the year if not throughout most years, the current nitrate and other nitrogen compounds input to the Bay through San Diego Creek as well as from other tributaries and local urban area street runoff and from possible groundwater input to the Bay are surplus compared to that needed to support the algal growth that is occurring in Bay waters. If this is the case, then the maximum degree of excessive fertilization of Upper Newport Bay waters is not now controlled by nitrogen input to the Bay. Further work needs to be done to examine this situation.
The information available shows that much of the nitrogen load to the Bay over the year has little or no influence on the excessive fertilization of the Bay because of the short residence time of tributary waters and their associated nitrogen loads in the Bay. The nitrogen compounds added during late fall, winter, and early spring do not generally cause excessive fertilization problems. They are flushed through the Bay before the excessive fertilization water quality problems begin in mid-spring. Further, there is little or no storage of nitrate or other forms of nitrogen added to the Bay during late fall, winter and early spring which would serve as the source of nitrogen for the growth of algae in late spring and early summer. The possible exception to this is for part of the particulate organic N which becomes incorporated into the Bay sediments. It is likely, however, that this source of nitrogen is an insignificant part of the algal available N that contributes to excessive fertilization water quality problems that occur during the summer months. This area needs review.
An area that has not yet been adequately investigated is the role of the high nitrate (30 to 60 mg/L NO3- - N) in the surficial groundwaters that enter San Diego Creek and its tributaries as well as possibly directly through subsurface flow into Upper Newport Bay as a source of nitrogen that is contributing to the excessive fertilization of Upper Newport Bay. It is possible that even with the Wetlands Water Supply Project being conducted by the IRWD (CH2M HILL, 1996), where part of the San Diego Creek waters that enter the Bay are passed through a wetlands area for nitrate removal (denitrification), the excessive fertilization problems of Upper Newport Bay will not be significantly affected by the current situation. It is unknown if the Wetlands Water Supply Project can remove sufficient nitrogen to reduce the excessive algal growth to the point where the public would perceive a significant improvement in Bay water quality.
The excessive fertilization of Upper Newport Bay is leading to increased concentrations of nitrogen and phosphorus compounds in Bay sediments. However, as discussed by Lee (1970), it is not possible to relate the concentrations of nutrients, such as nitrogen and phosphorus in sediments, to their significance as a source of nutrients that stimulate excessive planktonic and attached algal growth in a waterbody. The primary factors governing release of sediment-associated N and P to the overlying waters are hydrodynamic, related to mixing processes that occur between the sediment interstitial water and the water column. Further, the concentrations of nutrients or, for that matter, any other constituent are controlled by the flux of bulk constituents to the sediments. In areas where there is a high flux of watershed-derived erosional sediment to a waterbody, such as parts of Upper Newport Bay, the concentrations of nutrients in the sediments will be less due to the dilution effects of the erosional sediment added to the nitrogen and phosphorus incorporated into the sediments. This would lead to a lower concentration of N and P in the sediments for the same degree of eutrophication of a waterbody than would occur where the watershed-derived erosional sediment accumulation is less.
There is need to better understand nutrient (nitrogen and phosphorus) dynamics (aquatic chemistry), nutrient sources, and the factors controlling the excessive fertilization of Upper Newport Bay waters. This is especially important with particular reference to the amount of nitrogen and/or phosphorus inputs to the Bay during the critical periods of the year that leads to the excessive fertilization problems-use impairments. With this information it will be possible to better explore the development of nutrient control programs that will effectively control the eutrophication-related use impairments of Upper Newport Bay waters.
The nutrients in urban stormwater runoff that occur in late fall and winter will likely have little impact on algal growth in Upper Newport Bay. This arises from the fact that the aquatic plant nutrients present in this runoff are flushed through the Bay and therefore are not available to support algal growth during the spring, summer, and early fall when excessive fertilization problems occur within Upper Newport Bay.
As part of evaluating nutrient source impacts for Upper Newport Bay, particular attention should be given in these studies to what benefits, if any, could be expected from controlling urban area stormwater runoff-associated nutrients to Upper Newport Bay. It is possible that since the base flow inputs of San Diego Creek waters to the Bay derived from groundwater inputs as well as fugitive homeowners and commercial runoff waters that occur during spring and summer are such a dominant source of aquatic plant nutrients during the critical periods of the year, stormwater runoff-associated nutrient impacts are minor by comparison in adversely affecting eutrophication-related water quality in Upper Newport Bay. This is an area that needs detailed review as part the Santa Ana Regional Water Quality Control Board's current watershed-based studies as part of a TMDL for nutrients added to Upper Newport Bay.
If further studies show, as has been found in many other areas, that it is not possible to control the nutrient input to the Bay sufficiently to reduce the excessive fertilization that is occurring in the Bay, then aquatic life harvesting approaches should be considered as a means of improving the Bay's eutrophication-related water quality.
Dissolved Oxygen Depletion
Frequently, urban area and highway stormwater runoff monitoring programs will include measurement of biochemical oxygen demand (BOD) and/or COD as part of the monitoring of the runoff. While urban area and highway stormwater runoff can readily have measurable amounts of BOD, it is unlikely that this BOD will be of significance in affecting the oxygen resources of the receiving waters for the runoff. As discussed above, however, the aquatic plant nutrients added to a waterbody can be a significant source of nutrients that stimulate algal growth, which, in turn, leads to oxygen depletion in a waterbody's sediments and for a stratified waterbody, its hypolimnion.
If low DO water quality-use impairments are found in receiving waters, then specific studies need to be conducted to determine the origin of the chemical constituents that lead to the dissolved oxygen depletion. Such depletions can be caused by BOD, algal and aquatic plant photosynthesis-respiration, and chemical reactions between constituents in runoff waters or stirred into the water column during runoff events from the sediments, and the dissolved oxygen in the Bay waters and runoff waters.
In some situations, such as in shallow streams and bays, runoff waters will disturb the sediments so as to release sufficient quantities of ferrous iron and sulfide into the water column to cause depletion of the DO. Both ferrous iron and sulfide react rapidly with dissolved oxygen in the neutral pH range where the reactions take a few minutes to an hour or so, for completion. It has been reported (Crompton, 1996) that low DO problems occur in San Diego Creek associated with stormwater runoff events where the oxygen demand of the sediments is stirred into the water column during major runoff events. Algal and aquatic weed-caused depletions show a cyclic diel pattern, related to photosynthesis and respiration. BOD-caused depletions are slow-acting, taking several days for significant exertion of the oxygen demand associated with the bacterial respiration due to the use of organics as a source of food.
Site-specific evaluation of the oxygen resources of a waterbody can be conducted to determine if BOD associated with urban area and highway stormwater runoff is a significant contributor to the impairment of a waterbody's beneficial uses due to low DO. If such impacts are found, appropriate BMPs can be developed to control the BOD input to the waterbody from urban area and highway stormwater sources. The approach that would be followed would focus on the specific sources of the high BOD materials in the stormwater runoff, and controlling the input of the high BOD constituents at the source.
By examining the location, pattern of development, and the characteristics of the waterbody, it is possible to determine the cause of the dissolved oxygen depletion associated with a runoff event. In thermally or salinity-stratified waterbodies, it is possible, especially during the summer months, that low DO waters near the bottom could be mixed into the water column associated with runoff events. This can result in a fish kill due to low DO and the toxicity of hydrogen sulfide and ammonia. It does not appear, from the information available at this time, that this problem occurs in Upper Newport Bay, although it will be important to determine whether such a problem could develop associated with the deeper waters that will occur in the Bay after the proposed dredging of Bay sediments takes place. Once dredging is completed, new information available relative to this topic should be reviewed and the need for additional studies can be assessed.
It does not appear from past studies that there are significant dissolved oxygen depletion problems in Upper Newport Bay, although further studies need to be done to be certain the excessive fertilization of Bay waters does not lead to low dissolved oxygen problems as part of the diel changes in DO that occur in highly eutrophic waters. It is possible that the current IRWD studies of Upper Newport Bay may provide the data needed to evaluate this situation. Recently, Smythe (1997) has indicated that there are potential problems of this type that may be occurring in the Bay that need to be further investigated. These studies, however, will need to make measurements of DO during the early morning hours at various locations in the Bay, especially in areas with limited mixing, in order to determine if diel DO changes are occurring of potential significance to the Bay water quality. It should be noted, however, that short-term excursions below aquatic life DO standards associated with diel changes in DO do not appear to significantly impair the beneficial uses of a waterbody.
Litter Accumulation
Urban area and highway stormwater runoff can carry appreciable quantities of litter and debris that can impair the use of areas receiving the runoff. A key part of Evaluation Monitoring is determining whether litter and debris typically associated with urban area and highway stormwater runoff is present in the receiving waters to a sufficient extent to impair the uses of the waterbodies and their nearshore associated areas. If visual inspection of the receiving waters shows that areas of this type occur, improved litter and debris control can be implemented to eliminate the use impairment that is occurring associated with the materials carried in the runoff.
Litter is a significant cause of water quality deterioration-beneficial use impairment of Upper Newport Bay. From the information available, it is not clear that the primary sources of the litter that accumulate along the shore have been identified. Once this is done, then more effective litter control programs need to be initiated. Further, to effectively control the litter problem of the Bay, it will likely be necessary to initiate a more effective Bay nearshore litter removal program. Such programs can, if properly managed, control much of the adverse impacts of litter on the beneficial uses of a waterbody.
Oil and Grease Accumulation
This discussion focuses on the bulk effects of accumulated oil and grease, and does not address the aquatic life toxicity of petroleum hydrocarbons present in petroleum products. Those problems are considered under aquatic life toxicity for the water column and sediments. The stormwater runoff from urban areas and highways typically contains small amounts of petroleum hydrocarbons that can, under certain situations, cause water quality problems in receiving waters for the runoff.
In most situations, there is no need to treat the urban area and highway stormwater runoff to remove oil and grease since the small amounts of oil and grease ordinarily in this runoff do not cause significant water quality use impairments in the receiving waters. However, there are situations where petroleum hydrocarbons derived from oil and grease can be an important cause of water quality use impairments for urban area and highway stormwater runoff.
As part of an Evaluation Monitoring program, the receiving waters will be periodically visually examined to determine if there are areas where oil and grease from the urban area and highway stormwater runoff accumulate to a sufficient extent to be detrimental to aquatic life and other beneficial uses of the waterbody. Of particular concern would be fish spawning areas that accumulate sufficient amounts of petroleum hydrocarbons to be adverse to fish reproduction.
If the receiving waters are found to accumulate oil and grease from urban area and highway stormwater runoff to a sufficient extent to be adverse to the designated beneficial uses of the waterbody, a site-specific BMP can be developed which would control the input of oil and grease to the maximum extent practicable and, if necessary, treat the runoff waters to remove the oil and grease to the extent necessary to prevent adverse impacts. Before treatment is undertaken, however, attempts should be made to control the petroleum hydrocarbon contribution to the urban area and highway runoff based on source control activities.
At this time, no oil and grease accumulation problems have been identified in Upper Newport Bay. Examination of the Bay for oil and grease accumulation problems will be specifically conducted. If such problems are found, then the sources of the oil and grease that are accumulating in the area of concern should be identified through forensic studies and programs implemented to control the oil and grease at or near their source.
Sanitary Quality Impairment of Contact Recreation and Shellfish Harvesting
Urban area and highway stormwater runoff typically contains elevated concentrations of fecal coliforms and other organisms that are indicators of waterborne enteric pathogens. The sanitary quality (contact recreationCswimming, wading, and shellfish harvesting) of the receiving water for urban area and highway stormwater runoff can be adversely impacted by fecal coliforms (total coliforms for shellfish). The development of BMPs for urban area and highway stormwater runoff to address the control of enteric pathogenic organism indicators, such as total and/or fecal coliforms, should be based on finding excessive concentrations of these organisms in receiving waters for the runoff that impair the use of these waters.
Excessive concentrations are usually manifested in beach or swimming area closures/restrictions on shellfish harvesting. If such closures/restrictions of use are present in receiving waters for urban area and highway stormwater runoff, it is necessary to determine whether the runoff is, in fact, a significant contributor to the frequency and magnitude of closure/restrictions. If this situation is found, it will be important to determine whether there are connections between the sanitary sewerage system and the stormwater conveyance system that allow domestic wastewaters to enter the stormwater system during runoff periods.
Lee and Jones (1991c) have reported on the results of a study conducted in Lubbock, Texas where an evaluation was made of the impact of urban stormwater runoff-derived fecal coliforms and streptococci on recreational water quality in the Yellowhouse Canyon Lakes. These lakes are a chain of small lakes in a city park that receive appreciable stormwater runoff from the urban area. It was found that immediately after a stormwater runoff event, the sanitary quality of these lakes decreased to the point where they were considered unsafe for contact recreation, such as swimming. However, within a week to two weeks after the runoff event, the water in the lakes again met sanitary quality standards for contact recreation. During this period there was sufficient removal of the fecal indicator organisms through die-off and sedimentation to reduce their numbers below the fecal coliform standards.
Often today, there are attempts to distinguish between fecal indicator organisms derived from humans versus animals through determination of fecal coliform-fecal streptococci ratios in swimming area closure situations. If these ratios indicate that the fecal indicator organisms are derived from animal rather than human sources, it is sometimes determined that there is less need for the closure of the contact recreation area. However, justification for this approach is highly questionable based on the fact that Cryptosporidium is derived, at least in part, from cattle and possibly other animals. This organism is becoming recognized as an important cause of enteric disease associated with domestic water supplies and contact recreation (Lee and Jones-Lee, 1993b, 1995a). This is the organism that was responsible for causing approximately 400,000 people in Milwaukee, Wisconsin to become ill and about 100 people to die in a water supply waterborne epidemic in the spring of 1993. The source of this organism was believed to be from cattle where stormwater runoff waters containing cattle feces entered the Milwaukee raw water supply.
An area that is receiving increasing attention as a potential source of enteric pathogenic organisms is the use of reclaimed wastewaters for irrigation of ornamental shrubbery and other areas such as highway right-of-way shrubbery. As discussed by Lee and Jones-Lee (1995a,b), some regulatory agencies, such as the California Department of Health Services (CA DHS), allows the irrigation of ornamental shrubbery and golf courses with reclaimed domestic wastewaters that have not been adequately disinfected to control enteric viruses and cyst-forming protozoans such as Cryptosporidium. Disinfecting a domestic wastewater to just meet fecal coliform standards does not provide adequate disinfection to necessarily kill all the pathogenic enteric viruses and protozoan cysts. The use of partially treated reclaimed wastewaters to irrigate shrubbery along highways, parks, golf courses, etc. could lead to potential water quality problems associated with urban area and highway stormwater runoff.
The BMP for such problems would involve more appropriate disinfection of the reclaimed wastewaters before reuse. Lee and Jones-Lee (1995b) provided guidance on the water quality monitoring program that should be conducted to determine whether reclaimed domestic wastewaters represent important sources of fecal organisms that would represent a significant threat to the sanitary quality of a waterbody. They recommend that, in addition to monitoring for fecal coliforms, the monitoring program should include measurement of enteroviruses and cyst-forming protozoans such as Cryptosporidium and Giardia.
Blodgett (1989) reported that Upper Newport Bay was closed to shellfish harvesting due to bacterial contamination. It was noted that the fecal coliform inputs to Upper Newport Bay were more predominant during wet weather flows. No work was done to identify the specific sources of fecal coliforms that caused the shellfish harvesting closure. In addition to stormwater runoff, there is also discussion about the input of fecal coliforms from pleasure boats that discharge waste into the Bay. The primary concern is illegal disposal of sanitary wastes by boaters in the lower Bay and the lower part of Upper Bay which, through the tides, carries fecal indicator organisms into the upper parts of the Bay.
The Orange County Health Care Agency (OCHCA) has maintained a monitoring program for assessing the sanitary quality of Upper Newport Bay and San Diego Creek. The data obtained in this program during the 1990s have been reviewed as part of developing this report. These data show that, at times, the total coliforms and fecal coliforms in various parts of Upper Newport Bay exceed current sanitary quality standards for contact recreation and shellfish harvesting. While generally the highest total and fecal coliform values are found following stormwater runoff events, there are scattered high values during the dry period of the year. Overall it can be concluded that while the sanitary quality of Upper Newport Bay has improved from what it was in the 1970s and 1980s, there is still a threat of acquiring enteric disease from contact recreating in Upper Newport Bay.
The Orange County Health Care Agency's monitoring of San Diego Creek shows that normally the concentrations of total and fecal coliforms in the Creek waters are higher than those typically found in the Bay, indicating that the Creek is a source of fecal indicator organisms for the Bay. The specific sources of fecal indicator organisms for the Creek and Bay have not been investigated. One of the areas of potential concern is the overflow of sewage from blockage/failure of the municipal sewerage systems for the communities that are near the Bay. The OCHCA records a number of overflows of domestic wastewaters that occur to the Bay each year. Generally it has been found that there have been approximately 100 such events occurring each year in the 1990s. Some of these are small overflows, while others represent relatively large volumes of untreated domestic wastewaters entering the Bay or its tributaries.
The relative significance of the discharge of raw wastewaters to Upper Newport Bay due to failure of the sewerage systems for the communities near the Bay to other sources of fecal coliforms is not known at this time. It is possible that a comprehensive study of this issue could show that improving the reliability of the sewerage systems through increased inspection, maintenance, stand-by electrical power, pumps, etc. could reduce the frequency of excessive fecal and total coliform organisms in Upper Newport Bay.
Studies of dry weather storm sewer discharges to Santa Monica Bay (Haile et al., 1996) have shown that individuals undertaking contact recreation near where the storm sewer dry weather discharges enter the Bay have a higher incidence of disease than those who contact recreate in Santa Monica Bay waters away from where dry weather storm sewer discharges occur. Evidently, the dry weather storm sewer discharges contain pathogenic organisms that cause disease in those who contact recreate in the vicinity of the discharge.
Limited information is available on the enteroviruses and protozoan parasitic cyst organisms present in Bay waters. There can be little doubt that they are present and represent a potential threat to anyone using the Bay for contact recreation. The magnitude of this threat, however, is unknown at this time.
There is need to better understand the current sources of human and animal fecal pathogenic indicator organisms such as total and fecal coliforms, fecal streptococci, and other bacteria, as well as pathogens such as Cryptosporidium and selected enteroviruses for Bay waters to determine if it will be possible to improve the sanitary quality of Upper Newport Bay and thereby reduce the incidence of disease that is now occurring associated with contact recreation in the Bay.
Siltation-Excessive Sediment Accumulation and Turbidity
Suspended sediment derived from erosion or particulate matter associated with urban area and highway stormwater runoff can have an adverse impact on runoff receiving water quality. In addition to increasing the turbidity of the receiving waters, which can affect light penetration and the aesthetic quality of the water, sediment accumulation in the receiving waterbody can be adverse to aquatic organism habitat. Of particular concern is an adverse impact on fish spawning areas, changing the substrate for benthic organism development and changing the depth of the water so that rooted aquatic macrophytes are able to develop. Further, sediment accumulation can be sufficient in some instances to impact navigation.
One area of concern associated with suspended sediment-turbidity is an adverse impact on aquatic plant photosynthesis. Ordinarily, the turbidity associated with stormwater runoff events, while temporarily reducing the magnitude of photosynthesis due to decreased light penetration, does not significantly adversely impact the trophic status of the waterbody unless high levels of turbidity are present for extended periods of time, weeks or more in duration.
Suspended sediment is also of concern because of the potential for adverse effects on aquatic organisms' gills due to abrasion. As reported by Lee and Jones (1992), it has been found that aquatic organisms can tolerate high concentrations of suspended sediment for extended periods of time without significant adverse impacts. Aquatic organisms periodically experience high levels of suspended solids in many waterbodies due to storm or high flow-induced suspension of deposited sediments or erosion without adverse impacts on them.
It is important to distinguish between the physical impacts of sediments such as those discussed herein and the impacts due to chemical constituents associated with the sediments such as heavy metals, organics, etc. that are sorbed on the surface of the sediments. This issue has been reviewed by Lee and Jones-Lee (1996f). The physical impacts are those that are caused by the sediment and, in general, are independent of its chemical characteristics. Generally, it has been found that chemical constituents associated with sediments are in non-toxic, non-available forms and therefore do not, because of the chemical constituent, cause adverse impacts on water quality in the water column. For this reason, the U.S. EPA (1995b) has determined that ambient water heavy metals should be regulated based on the dissolved forms of the heavy metals. This situation has important implications for managing heavy metals in urban area and highway stormwater runoff since, in general, these metals are in particulate forms.
Blodgett (1989) reported that erosion from the watershed and the resultant siltation in Upper Newport Bay are continuing threats to the beneficial uses of the Bay. He indicated that San Diego Creek is responsible for 94 percent of the sediment delivered to Upper Newport Bay. It is likely that Bonita Creek, a tributary of San Diego Creek located just upstream of Upper Newport Bay, has been a significant historical contributor of sediment. The Bonita Creek watershed contains the Coyote Canyon Landfill, now closed, which has had a high sediment yield potential due to continuous grading operations. The proximity of the landfill to the Bay is also important in that the sediment delivery ratio would be relatively high.
Upper Newport Bay is experiencing excessive sediment accumulation due to erosion from its watershed. This sediment accumulation causes shoaling (reduction of water depth), which interferes with navigation. It also changes the depth of the Bay water and, thereby, alters the aquatic plant habitat so that macrophytes encroach into the open water areas. Extensive studies have been conducted on the sources of the erosional sediment, and programs have been implemented to control the erosion at the source as well as through trapping before San Diego Creek water enters Upper Newport Bay. The OCPFRD has an ongoing evaluation of sediment control program effectiveness and makes changes in the program as funds are available.
Impairment of Domestic Water Supply Water Quality
The effect of urban area and highway stormwater runoff on domestic water supply water quality needs to be considered from the two perspectives of surface and groundwater-based water supplies. The basic issue is whether urban area and highway stormwater runoff introduces new constituents in sufficient amounts to be a significant threat to domestic and other water supply water quality. Both human health (hazardous chemicals and pathogenic organisms) and aesthetic quality should be considered, including taste and odor producing compounds, hardness, TDS, and other constituents that can impact domestic water supply water quality. In situations where there is already appreciable urban area and highway stormwater runoff contributed to a domestic water supply, the issue then becomes one of whether the additional load of urban area and highway stormwater runoff-derived constituents represents a significant additional load that either causes the water utility to start treating to remove the constituents or to experience increased treatment costs to remove the additional load of constituents.
For domestic water supplies that are based on groundwater sources, the issue becomes one of assessing the potential for urban area and highway stormwater runoff-derived constituents to adversely impact the groundwater that is recovered from the area where urban area and highway stormwater runoff-derived constituents are recharged into the aquifer system. While many aquifers have an appreciable ability to remove chemical constituents in recharged waters through soil aquifer treatment, there is a potential for build-up of persistent chemicals and/or transformation products of treated chemicals within the aquifer system. As discussed by Lee and Jones-Lee (1993c, 1994b) concern must also be given to whether constituents in recharged waters could cause the aquifer to become contaminated to a sufficient degree to lead to the need for aquifer remediation in a "superfund"-like program.
Since chemical constituents and pathogenic organisms in urban area and highway stormwater runoff are threats to domestic water supply raw water quality, it will be important to evaluate whether stormwater runoff from these areas is significantly adverse to a water utility=s use of a waterbody as a raw water supply. For most water quality parameters, the Evaluation Monitoring approach discussed herein, which focuses on defining real water quality problems of significance to aquatic life and recreational uses of waters, will, in general, detect significant water quality problems for domestic water supplies. There are, however, some exceptions to this situation.
Certain chemical constituents and pathogenic organisms in waters are of concern because of their impact on raw water supply water quality. Examples would be low molecular weight organics that are potential carcinogens that do not bioaccumulate in fish tissue to a sufficient extent to cause health hazards for human consumption or consumption by higher trophic level organisms. Chemicals of this type are the volatile organic compounds (VOCs--low molecular weight chlorinated solvents and volatile organics such as benzene). Ordinarily these types of chemicals are not considered to be the cause of water quality problems in stormwater runoff from urban areas and highway due to their high volatility since they are rapidly lost to the atmosphere. An important exception to this situation is the recent finding of MTBE (methyl tertiary butyl ether), a gasoline additive, in surface and groundwaters. While the full understanding of the sources, water quality significance, and fate of MTBE is not known at this time, it appears from the information available that its introduction into gasoline as an additive to reduce air pollution is causing pollution of surface and groundwaters that may be associated with stormwater runoff from highways and streets. Tratnyek et al. (1997) organized a session of the American Chemical Society, Environmental Division held in April 1997 devoted to environmental fate and effects of gasoline oxygenates. MTBE has been found in over 51 public water supply systems. Further information on the potential significance of MTBE as a water pollutant is available in the various papers from the Tratnyek et al. (1997) ACS session.
Arsenic is a chemical that could become important in affecting domestic water supplies, but not other beneficial uses of waterbodies. Depending on the arsenic concentration the U.S. EPA selects as the new Maximum Contaminant Level (MCL), arsenic could become one of the most important parameters influencing raw water quality. It is of concern because of its potential to cause cancer and other diseases in people. Some stormwater runoff studies have shown arsenic from urban areas to be at concentrations above some of the U.S. EPA=s proposed MCLs. In time, considerable attention will be given to specific sources of arsenic that cause a waterbody to have concentrations of arsenic that require treatment for use of the water for domestic water supply purposes. When this occurs, the sources of arsenic in urban area and highway stormwater runoff will need to be determined to ascertain if the elevated concentrations of arsenic in the runoff can be controlled at the source.
Another group of chemicals of potential concern are the trihalomethane precursors (dissolved organic carbon [DOC]) that are derived from the decay of terrestrial and some forms of aquatic vegetation as well as some wastewater sources. Eventually, the U.S. EPA and state regulatory agencies will be attempting to control sources of DOC for waterbodies in an effort to reduce the DOC content of the raw water. While various types of land use have differing DOC export coefficients (g of DOC/m2/yr), insufficient information is available at this time to indicate that stormwater runoff from highways and urban areas is a particularly significant source of DOC. Further information on evaluation and management of domestic water supply raw water quality is found in the review by Lee and Jones (1991d).
In many areas, urban area and highway stormwater runoff recharges groundwater basins. The chemical constituents and pathogenic organisms in the runoff can be a threat to groundwater quality. While, in most instances, the constituents in urban area and highway stormwater runoff will not significantly alter the potential for the receiving waters to impair the uses of groundwater, there may be unusual situations where groundwater quality could be impaired by constituents in urban area and highway stormwater runoff. Typically, the additional loads of constituents in runoff waters are such that they do not significantly change the concentrations of constituents of concern for groundwater quality through the recharged waters. Further, many of the constituents with elevated concentrations in urban area and highway stormwater runoff are in particulate forms that are removed from the recharge waters as the receiving waters plus the runoff waters percolate into the aquifer system.
Some of the dissolved constituents in urban area and highway stormwater runoff will be sorbed onto vadose zone (unsaturated) and saturated zone solids of the aquifer and thereby not cause groundwater quality use impairment. The aquifer mobile fractions of the chemical constituents in the runoff waters such as nitrate, chloride, sodium, etc. are normally present in urban area and highway stormwater runoff waters at concentrations that do not represent threats to groundwater quality. An exception to this situation is detention/infiltration basins where the constituents in the urban area and highway stormwater runoff are not diluted in the receiving waters for the runoff. Under these conditions, it is possible to build up sufficient concentrations of some chemical constituents in the recharge waters to threaten groundwater quality. An area of particular concern is stormwater runoff that contains chemicals used for de-icing of streets and highways. High concentrations of sodium and/or calcium chloride can occur in these waters.
At a location where urban area and highway stormwater runoff is recharged directly or is a significant component of receiving waters that recharge an aquifer, such as in areas where infiltration of stormwater is used for stormwater runoff management, a site-specific evaluation should be made to determine whether the recharge waters are adversely impacting the quality of the waters in the aquifer. Typically, this is best done by sampling the groundwaters immediately under the recharge areas and down groundwater gradient of the recharge point. If excessive concentrations of chemical constituents are found in the groundwaters that can be attributed to recharge, evaluations should be made as to whether these constituents are derived to a significant extent from urban area and highway stormwater runoff.
A special area of concern with respect to groundwater pollution by stormwater runoff is the potential for accidental spills of chemicals to pollute aquifer systems. It is important, as part of developing an accidental spill contingency plan, to be able to contain the spill as much as possible in areas in which there are low-permeability aquifer materials or paved surface as a barrier between the spilled chemicals and the water table. Further, in the event of a spill, those responsible for managing urban area and highway stormwater runoff should be prepared to quickly begin remediation of the contaminated parts of the aquifer to prevent the spread of the spilled chemicals through the unsaturated-vadose zone and into the saturated groundwaters.
All groundwater-based water utilities should be monitoring the characteristics of the recharged waters near the point of recharge to detect incipient water quality problems. Urban area and highway stormwater runoff-derived constituents of potential concern should be added to the list of aquifer-based monitored parameters. Similarly, surface-based water supply systems managers should be conducting a detailed monitoring program of the raw water quality. If any of the urban area and highway stormwater runoff-derived constituents represent a threat to the surface water quality, groundwater or aquifer quality, then site-specific BMPs should be developed to control the constituents at the source, or to treat the urban area and highway stormwater runoff to protect the water supply water quality.
Since Upper Newport Bay is estuarine/marine, it is not a domestic water supply source. While San Diego Creek and its tributaries are freshwater and could, at some locations, recharge groundwaters, the groundwaters in some areas of the San Diego Creek watershed are polluted by past agricultural and industrial/military activities leading to high TDS, nitrate, and in some areas chlorinated solvents. It does not appear, however, that the groundwater chlorinated solvent problems in the Upper Newport Bay watershed are adverse to the beneficial uses of San Diego Creek or Upper Newport Bay.
It is not clear at this time whether the poor water quality of San Diego Creek due to groundwater discharge to the creek, with elevated TDS and nitrate, has or is currently polluting groundwaters in other areas due to creek and tributary recharge of the groundwater system. At this time, there is relatively poor understanding of surface water-groundwater interactions and associated water quality issues in the Upper Newport Bay watershed. This area needs attention that could lead to an improvement in San Diego Creek water quality as well as water quality in Upper Newport Bay. It could be that the discharge of the currently polluted groundwaters to the creek from specific areas could be controlled through pump-and-treat operations and thereby improve creek and Upper Newport Bay water quality.
Overall Upper Newport Bay Water Qualities
Overall, Upper Newport Bay is experiencing significantly impaired water quality due to:
This project was supported by Silverado Constructors, Irvine, California.
Ankley, G.T., Schubauer-Berigan, M.K., Dierkes, J.R. and Lukasewycz, M.T., ASediment Toxicity Identification Evaluation: Phase I (Characterization), Phase II (Identification) and Phase III (Confirmation) Modifications of Effluent Procedures,@ Draft report, U.S. EPA, Environmental Research Laboratory-Duluth, Technical Report 08-91, Duluth, MN (1991).
Bailey, H.C., DiGiorgio, C. and Hinton, D.E., ANewport Bay Watershed Toxicity Study - Final Report,@ Submitted to Santa Ana Region-California Regional Water Quality Control Board, Department of Medicine, School of Veterinary Medicine, University of California--Davis, Davis, CA (1993).
Bailey, H.C., DiGiorgio, C. and Kroll, K., ADevelopment of Procedures for Identifying Pesticide Toxicity in Ambient Waters: Carbofuran, Diazinon, Chlorpyrifos,@ Environmental Toxicology and Chemistry, 15:(6) 837-845 (1996).
Blodgett, P.L., ANewport Bay Clean Water Strategy-A Report and Recommendation for Future Action,@ Submitted to California State Senate, Santa Ana Region-California Regional Water Quality Control Board, Riverside, CA, September (1989).
CH2M HILL, AAddendum to Environmental Impact Report, Irvine Ranch Water District Wetlands Water Supply Project,@ Report prepared for the Irvine Ranch Water District, Irvine, CA by CH2M HILL, Santa Ana, CA, January (1996).
Connor, V., "Pesticide Toxicity in Urban Storm Runoff," Presentation to the Central Valley Regional Water Quality Control Board, Sacramento, CA, June (1995).
Cooper, A., ADiazinon in Urban Areas,@ Report of the Regional Water Quality Control Plant, Palo Alto, CA, August (1996).
Cox, C., AConcentrations of Selected Radionuclides and Chemicals in Fish, Sediment, and Water Collected From the Putah Creek Near the Former Laboratory for Energy-Related Health Research, Davis, CA,@ Report for Agency for Toxic Substances and Registry by the National Air and Radiation Environmental Laboratory, U.S. Environmental Protection Agency/NAREL, Montgomery, AL, March (1997).
Crisp, T.M. and others, ASpecial Report on Environmental Endocrine Disruption: An Effects Assessment and Analysis,@ EPA/630/R-96/012. U.S. Environmental Protection Agency, Risk Assessment Forum, Washington, D.C., February (1997).
Crompton, C., County of Orange Public Facilities Department, personal communication to G. Fred Lee (1996).
Domagaiski, J.L., ANonpoint Sources of Pesticides in the San Joaquin River, California: Input from Winter Storms, 1992-93,@ U.S. Geological Survey Open-File Report 95-165, Sacramento, CA (1995).
DPR, AAnnual Pesticide Use Report,@ Orange County, Department of Pesticide Regulations, Sacramento, CA (1994).
Dragun, J. and Chiasson, A., AElements In North American Soils,@ Hazardous Materials Control Resources Institute, Rockville, MD (1991).
Environment Canada, AChromium and its Compounds,@ Report No. PSL-39, Environment Canada, Hull, Quebec (1995).
Fetterolf, C., Great Lakes Fisheries Commission, personal communication to G. Fred Lee, retired (1996).
Foe, C., AEvaluation of the Potential Impact of Contaminants on Aquatic Resources in the Central Valley and Sacramento-San Joaquin Delta Estuary,@ Central Valley Regional Water Quality Control Board, Sacramento, CA, June (1995a).
Foe, C., AInsecticide Concentrations and Invertebrate Bioassay Mortality in Agricultural Return Water From the San Joaquin Basin,@ Central Valley Regional Water Quality Control Board. Sacramento, CA, December (1995b).
Gerstenberg, G., AManagement Plan Upper Newport Bay Ecological Reserve,@ State of California Resource Agency Department of Fish and Game, Newport Beach, CA (undated).
Haile, R.W. et al., AAn Epidemiological Study of Possible Adverse Health Effects of Swimming in Santa Monica Bay," Final Report to Santa Monica Bay Restoration Project, Regional Water Quality Control Board, Los Angeles Region, Monterey Park, CA, May (1996).
Hansen & Associates, AIdentification and Control of Toxicity in Storm Water Discharges to Urban Creeks, Final Report,@ Prepared for Alameda County Urban Runoff Clean Water Program, Hayward, CA, March (1995).
IRWD, Data from Upper Newport Bay Monitoring Program, Irvine Ranch Water District, Irvine, CA (1996).
Jones, R.A. and Lee, G.F., ARecent Advances in Assessing the Impact of Phosphorus Loads on Eutrophication-Related Water Quality,@ Journ. Water Research 16:503-515 (1982).
Jones, R.A. and Lee, G.F. AEutrophication Modeling for Water Quality Management: An Update of the Vollenweider-OECD Model,@ World Health Organization Water Quality Bulletin 11(2):67-74, 118 (1986).
Katznelson, R. and Mumley, T., ADiazinon in Surface Waters in the San Francisco Bay Area: Occurrence and Potential Impact," Report to California State Water Resources Control Board and Alameda Countywide Clean Water Program, Oakland, CA, June (1997).
Kuivila, K.M., ADiazinon Concentrations in the Sacramento and San Joaquin Rivers and San Francisco Bay, California, February 1993,@ U.S. Geological Survey Water Facts Sheet, Open-File Report 93-440, Sacramento, CA (1993).
Kuivila, K.M., and Foe, C.G., AConcentrations, Transport and Biological Effects of Dormant Spray Pesticides in the San Francisco Estuary, California,@ Environmental Toxicology and Chemistry, 14:1141-1150 (1995).
Lee, G.F., "Factors Affecting the Transfer of Materials Between Water and Sediments," University of Wisconsin Eutrophication Information Program, Literature Review No. 1, 50 pp (1970).
Lee, G.F. "Unreliability of Sea Urchin Fertilization Test for Regulating Aquatic Life Toxicity," Comments submitted to California State Water Resources Control Board, Sacramento, CA (1991).
Lee, G.F. and Jones, R.A.., "An Approach for Assessing the Water Quality Significance of Chemical Contaminants in Urban Lakes," Proc. Urban Storm Water and Combined Sewer Overflow Impact on Receiving Water Bodies Symposium, EPA 600/9-80-056, U.S. EPA-Cincinnati, OH, pp. 32-57 (1980).
Lee, G.F. and Jones, R.A., "Detergent Phosphate Bans and Eutrophication," Environ. Sci. & Technol. 20:330-331 (1986).
Lee, G.F. and Jones, R.A., "Determination of the Nutrient Limiting Maximum Algal Biomass in Water Bodies," Report G. Fred Lee & Associates, El Macero, CA, (1988a).
Lee, G.F. and Jones, R.A., "Study Program for Development of Information for the Use of Vollenweider-OECD Eutrophication Modeling in Water Quality Management," Report G. Fred Lee & Associates, El Macero, CA (1988b).
Lee, G.F. and Jones, R.A., "Suggested Approach for Assessing Water Quality Impacts of Urban Storm water Drainage," Proc. Symposium on Urban Hydrology, American Water Resources Association AWRA Technical Publication Series TPS-91-4, AWRA, Bethesda, MD, pp. 139-151 (1991a).
Lee, G.F. and Jones, R.A., "Effects of Eutrophication on Fisheries," Review in Aquatic Sciences, 5:287-305, CRC Press, Boca Raton, FL (1991b).
Lee, G.F. and Jones, R.A., "Indirect Reuse of Domestic Wastewater for Recreational Lakes: Evaluation of the Sanitary Quality of the Yellowhouse Canyon Lakes, Lubbock, Texas," Proc. American Water Resources Association Symposium: Water Supply and Water Reuse: 1991 and Beyond, San Diego, CA, pp. 435-449 (1991c).
Lee, G.F. and Jones, R.A., "Regulating Drinking Water Quality at the Source," Proc. University of California Water Resources Center Conference: Protecting Water Supply Water Quality at the Source, Sacramento, CA, 39 pp (1991). Also published in part as: "Managing Delta Algal Related Drinking Water Quality: Tastes and Odors and THM Precursors," pp. 105-121 (1991d).
Lee, G.F. and Jones, R.A., "Water Quality Aspects of Dredging and Dredged Sediment Disposal," In: Handbook of Dredging Engineering, McGraw Hill, pp. 9-23 to 9-59 (1992)
Lee, G.F. and Jones-Lee, A., "Importance of Considering Soil-Lead in Property Site Assessments," Presented at National Ground Water Association Conference, "Environmental Site Assessments: Case Studies and Strategies," Orlando, FL. 23pp, available as report G. Fred Lee & Associates, El Macero, CA, August (1992).
Lee, G.F., Jones, R.A. and Rast, W., "Availability of Phosphorus to Phytoplankton and Its Implication for Phosphorus Management Strategies," In: Phosphorus Management Strategies for Lakes, Ann Arbor Press, Ann Arbor, MI, pp. 259-308 (1980)
Lee, G.F. and Jones-Lee, A., "Sediment Quality Criteria: Numeric Chemical- vs. Biological Effects-Based Approaches," Proc. Water Environment Federation National Conference, Surface Water Quality & Ecology, Anaheim, CA, pp. 389-400, October (1993a).
Lee, G.F. and Jones-Lee, A., "Public Health Significance of Waterborne Pathogens in Domestic Water Supplies and Reclaimed Water," Report to State of California Environmental Protection Agency Comparative Risk Project, Berkeley, CA, 27 pp (1993b).
Lee, G.F. and Jones-Lee, A., "Water Quality Aspects of Incidental and Enhanced Groundwater Recharge of Domestic and Industrial Wastewaters-An Overview," Proc. Symposium on Effluent Use Management, TPS-93-3, pp. 111-120, American Water Resources Association, Bethesda, MD (1993c).
Lee, G.F. and Jones-Lee, A., "Contaminated Dredged Sediment Disposal Criteria," Proc. ASCE "Dredged 94" Second International Conference on Dredging and Dredged Materials Placement, American Society of Civil Engineers, pp. 121-130, Washington, D.C. (1994a).
Lee, G.F. and Jones-Lee, A., "Water Quality Aspects of Groundwater Recharge: Chemical Characteristics of Recharge Waters and Long-Term Liabilities of Recharge Projects," Proc. American Society of Civil Engineers Second International Symposium on Artificial Recharge. Orlando, FL, pp. 502-511 (1994b).
Lee, G.F and Jones-Lee, A., "Public Health and Environmental Safety of Reclaimed Wastewater Reuse," Proc. Seventh Symposium on Artificial Recharge of Groundwater, University of Arizona Water Resources Research Center, Tucson, AZ, pp.113-128 (1995a).
Lee, G.F. and Jones-Lee, A., "Monitoring Reclaimed Domestic Wastewater Usage on Public Parkland to Reduce Risks," Water Engineering & Management, 142:28-29,37 (1995b).
Lee, G.F. and Jones-Lee, A., "Planktonic Algal Toxicity Testing in Regulating Point and Non-Point Discharges and its Implications for Use of Dissolved Metal Criteria/Standards," Submitted for publication, Water Environment Federation Water Environment & Technology (1996a).
Lee, G.F. and Jones-Lee, A., "Summary of Issues Pertinent to Regulating Bioaccumulatable Chemicals," Report of G. Fred Lee & Associates, El Macero, CA, September (1996b).
Lee, G.F. and Jones-Lee, A., "'Co-Occurrence' in Sediment Quality Assessment," Report G. Fred Lee & Associates, El Macero, CA, February (1996c).
Lee, G.F. and Jones-Lee, A., "Evaluation of the Water Quality Significance of the Chemical Constituents in Aquatic Sediments: Coupling Sediment Quality Evaluation Results to Significant Water Quality Impacts," In: WEFTEC '96, Surface Water Quality and Ecology I & II, Vol 4, pp 317-328, Proc. Water Environ. Fed. Annual Conference (1996d).
Lee, G.F. and Jones-Lee, A., "Assessing Water Quality Impacts of Storm Water Runoff," North American Water & Environment Congress, Published on CD-ROM, Amer. Soc. Civil Engr., New York, 6pp (1996e).
Lee, G.F. and Jones-Lee, A., "Significance of Eroded Suspended Sediment-Associated Constituents," Land and Water, 40(1):19-23 (1996f).
Lee, G.F. and Jones-Lee, A., "Chromium Speciation: Key to Reliable Control of Chromium Toxicity to Aquatic Life," Environmental Division poster session, American Chemical Society National Meeting, San Francisco, CA, April (1997a).
Lee, G.F. and Jones-Lee, A., "Development and Implementation of Evaluation Monitoring for Storm Water Runoff Water Quality Impact, Assessment and Management," Report of G. Fred Lee & Associates, El Macero, CA, June (1997b).
Lee, G.F. and Jones-Lee, A., "Lead as a Stormwater Runoff Pollutant," Report of G. Fred Lee & Associates, El Macero, CA, June (1997c).
Lee, G.F. and Taylor, S., "Aquatic Life Toxicity in Stormwater Runoff to Upper Newport Bay, Orange County, California: Initial Results," Report to Silverado, Irvine, CA, Submitted by G. Fred Lee & Associates, El Macero, CA, June (1997).
MBC, "Biological Resources Assessment of the Temporary Partial Diversion of Water from San Diego Creek," Report to CH2M HILL prepared by Marine Biological Consultants Applied Environmental Sciences, Costa Mesa, CA, December (1995).
McConnell, L.L., Nelson, E., Rice, C.P., Baker, J.B., Johnson, W.E., Harman, J.A. and Bialek, K., "Chlorpyrifos in the Air and Surface Water of Chesapeake Bay: Predictions of Atmospheric Deposition Fluxes," Environmental Science Technology, 31(5):1390-1398, ES960614x (1997).
Mount, D., ASCI Corp., Duluth, MN, Personal communication to G. Fred Lee, May (1997).
NAS/NAE, "Water Quality Criteria of 1972," National Academies of Science and Engineering, U.S. EPA, EPA/R3-73-033, Washington, D.C. (1973).
OCEMA, "Hydrology for Santa Ana-Delhi Channel and Principal Tributaries," Orange County Environmental Management Agency, Santa Ana, CA (1985).
OCEMA, "Report of Waste Discharge," Orange County Environmental Agency, Orange County NPDES Storm water Program, Submitted to San Diego and Santa Ana Regional Water Quality Control Boards, Santa Ana, CA, December (1994).
OCEMA, "Data Report for Storm Water Runoff Water Quality Monitoring Program," Orange County Environmental Agency, Santa Ana, CA (1996).
Olson, T.M. and Martinez, A., "Newport Bay Basin Update Plan Study - Draft Final Report," Submitted to Santa Ana Region - California Regional Water Quality Control Board, Department of Civil Engineering, University of California, Irvine, CA, June (1993).
Orange County Health Care Agency, "Environmental Studies In Newport Bay," Environmental Health Division Report, Santa Ana, CA, June (1978).
Pinza, M.R, Kohn, N.P., Ohlrogge, S.F., Ferguson, C.J., and Word, J.Q., "Reducing the Effects of Total Ammonia in 10-Day Sediment Toxicity Tests with the Amphipod, Rhepoxynius Abronius," Proc. Environmental Toxicology and Risk Assessment: Biomarkers and Risk Assessment, Fifth Volume, ASTM STP 1306, American Society for Testing and Materials, West Conshohocken, PA (1996).
Pitt, R.E. and Field, R., "Hazardous and Toxic Wastes Associated with Urban Storm water Runoff," Proc. of the Sixteenth Annual RREL Hazardous Waste Research Symposium, EPA/600/9-90 037, U.S. Environmental Protection Agency, Office of Research and Development, Cincinnati, OH, pp 274-289 (1990).
Powell, S.J. and Leyva, J.J., "Herbicide Runoff in Rainwater from Treated Highway Rights-of-Way," Presentation at the Northern California SETAC annual meeting, California Department of Pesticide Regulation, Sacramento, CA (1994).
Rasmussen, D., personal communication to G. Fred Lee, California Water Resources Control Board, Sacramento, CA (1996).
Rast, W. and Lee, G.F., "Nutrient Loading Estimates for Lakes," J. Environ. Engr. Div. ASCE 109:502-517 (1983). See also, "Nutrient Estimates for Lakes," Journ. Environ. Engr. 110:722-724 (1984).
Salazar, M., Salazar, S.M., Paynter, K. and Gaffney, P., "Using Transplanted Bivalves to Assess Oil Exposure and Effects in Delaware Bay," Presented at Fifth symposium on Env. Tox. and Risk Assessment: Biomarkers and Risk Assessment, ASTM (1995).
SARWQCB, "Water Quality Control Plan, Santa Ana River Basin," Regional Water Quality Control Board, Santa Ana Region, Riverside, CA (1995).
SFRWQCB, "Contaminant Levels in Fish Tissue from San Francisco Bay," Final Report, San Francisco Regional Water Quality Control Board, State Water Resources Control Board, California Department of Fish and Game, June (1995).
SFRWQCB, "Survey of Storm Water Runoff for Dioxins in the San Francisco Bay Area," California Regional Water Quality Control Board San Francisco Bay Region, Oakland, CA, February (1997).
Silverado, "Runoff Management Plan for Eastern Transportation Corridor," Vol. 2, Technical Appendix C, Silverado Constructors, Irvine, CA, February (1997).
Simons, Li and Associates, "Project Report for San Diego Creek, Facility F05 From Jamboree Road to Jeffrey Road, Phase I: Hydrologic, Hydraulic and Sedimentation Study," Orange County Environmental Management Agency, Santa Ana, CA, October (1987).
Smythe, H., "San Diego Creek Watershed Nitrogen Study: Final Report," Santa Ana Regional Water Quality Control Board, Riverside, CA, November (1990).
Tettemer and Associates, "Flood Control Master Plan for San Diego Creek," Agency Supplied Document 23F, prepared by J. M. Tettemer and Associates, Costa Mesa, CA, April (1989).
Tratnyek, P.G., Bauman, B.J. and Zogorski, J.S., "Environmental Fate and Effects of Gasoline Oxygenates," Division of Environmental Chemistry, American Chemical Society, Preprints of Extended Abstracts, national meeting, 37:366-432, April (1997).
U.S. EPA, "Quality Criteria for Water 1986," EPA 440/5086-001, U.S. Environmental Protection Agency, Office of Water Regulations and Standards. Washington, D.C. (1987).
U.S. EPA, "Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories, Vol. I, Fish Sampling and Analysis," EPA 823-R-93-002, U.S. Environmental Protection Agency, Office of Water, Washington, D.C. (1993a).
U.S. EPA, "Wildlife Criteria Portions of the Proposed Water Quality Guidance for the Great Lakes System," EPA-822-R-93-006, U.S. Environmental Protection Agency, Office of Water and Office of Science and Technology, Washington, D.C. (1993b).
U.S. EPA, "Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories, Vol. II, Risk Assessment And Fish Consumption Limits," EPA 823-B-94-004, U.S. Environmental Protection Agency, Office of Water, Washington, D.C., June (1994).
U.S. EPA, "Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories, Vol. IV, Risk Communication," EPA 823-R-95-001, U.S. Environmental Protection Agency, Office of Water, Washington, D.C. (1995a).
U.S. EPA, "Stay of Federal Water Quality Criteria for Metals; Water Quality Standards; Establishment of Numeric Criteria for Priority Toxic Pollutants; States' Compliance Revision of Metals Criteria; Final Rules," Federal Register, Volume 60, Number 86, pp 22228-22237, Washington, D.C., May 4 (1995b).
U.S. EPA/COE, "Evaluation of Dredged Material Proposed for Ocean Disposal: Testing Manual," EPA-503/8-91/001, U.S. Environmental Protection Agency/Corps of Engineers, Washington, D.C. (1991).
U.S. EPA/COE, "Evaluation of Dredged Material Proposed for Freshwater Disposal: Testing Manual," Draft, U.S. Environmental Protection Agency/Corps of Engineers, Washington, D.C. (1994).
USGS, "Diazinon Concentrations in the Sacramento and San Joaquin Rivers and San Francisco Bay, California, February 1993," U.S. Geological Survey, Water Fact Sheet, Open File Report 93-440, Department of the Interior, Sacramento, CA (1993).
Waller, W.T., Acevedo, M.F., Morgan, E.L., Dickson, K.L., Kennedy, J.H., Ammann, L.P., Allen, H.J. and Keating, P.R., "Biological and Chemical Testing in Storm Water," In: Stormwater NPDES Related Monitoring Needs, H. C. Torno (ed.), Proc. Engineering Foundation Conference, August 1994, ASCE, New York (1995).
WRCB, "Bay Protection and Toxic Cleanup Program Quality Assurance Project Plan," Water Resources Control Board, Sacramento, CA, July (1994).
WRCB, "Toxic Substances Monitoring Program (TSMP), Data Base Description," State Water Resources Control Board, Sacramento, CA, Revised September (1995).
WRCB, "California State Mussel Watch, Data Base Description," State Water Resource Control Board, Sacramento, CA, Revised May (1996a).
WRCB, "Upper Newport Bay Data," Bay Protection Toxic Cleanup Program, Water Resources Control Board, Sacramento, CA (1996b).
Wright, T., "Evaluation of Dredged Material for Open-Water Disposal: Numerical Criteria or Effects-Based?" In: Handbook of Dredging Engineering, McGraw-Hill, New York, pp. 9.59-9.67 (1992).
SUPPLEMENTAL REFERENCES
Baker, J.L. et al. (Eds.), Aquatic Dialogue Group: Pesticide Risk Assessment and Mitigation, SETAC Press, Pensacola, FL (1994).
Bay, S., Greenstein, D., Jirik, A., Zellers, A., Lau, S-L. and Noblet, J., AToxicity of Stormwater Runoff from the Santa Monica Bay (California) Watershed,@ Presented at the 17th annual SETAC Meeting, November 17-21 (1996)
de Vlaming, V., AAre the Results of Single Species Toxicity Tests Reliable Predictors of Aquatic Ecosystem Community Responses? A Review,@ Report, Monitoring and Assessment Unit Division of Water Quality, State Water Resources Control Board, Sacramento, CA (1995).
Denton, D., "Region IX WET Update," U.S. EPA Region IX, San Francisco, CA, December (1995).
Grothe, D.R., Dickson, K.L. and Reed-Judkins, D.K., Whole Effluent Toxicity Testing: An Evaluation of Methods and Prediction of Receiving System Impacts, SETAC Press Pensacola, FL (1996).
Herricks, E.E., (ed.). Stormwater Runoff and Receiving Systems: Impact, Monitoring, and Assessment, CRC Press, Inc., Boca Raton, FL (1995).
Jones-Lee, A., and Lee, G.F., AAchieving Adequate BMP=s for Storm Water Quality Management,@ Proc. 1994 National Conference on Environmental Engineering, Critical Issues in Water and Wastewater Treatment, American Society of Civil Engineers, New York, NY, pp. 524-531, July (1994).
Lee, G.F., "Comments on: The Santa Monica Bay Restoration Plan, September 1994, for Storm Water Runoff Water Quality Management," Report G. Fred Lee & Associates, El Macero, CA (1995).
Lee, G.F. and Jones, R.A., "Interpretation of Chemical Water Quality Data," In: Aquatic Toxicology, ASTM STP 667, ASTM, Philadelphia, pp 302-321 (1979).
Lee, G.F., Jones, R.A. and Newbry, B.W., "Water Quality Standards and Water Quality," Journ. Water Pollution Control Fed. 54:1131-1138 (1982)
Lee, G.F. and Jones-Lee, A., "Guidance for Conducting Water Quality Studies for Developing Control Programs for Toxic Contaminants in Wastewaters and Stormwater Runoff," Report of G. Fred Lee & Associates, El Macero, CA, 30 pp, July (1992).
Lee, G.F. and Jones-Lee, A., "Water Quality Impacts of Stormwater-Associated Contaminants: Focus on Real Problems," Report by G. Fred Lee & Associates, El Macero, CA, 45pp, July (1993).
Lee, G.F. and Jones-Lee, A, "Water Quality Impacts of Storm water-Associated Contaminants: Focus on Real Problems - Condensed Version," Proc. First International IWQA Specialized Conference on Diffuse Pollution: Sources, Prevention, Impact and Abatement, Chicago, IL, pp. 231-240, September (1993).
Lee, G.F. and Jones-Lee, A., "Deficiencies in Stormwater Quality Monitoring," Proc. Engineering Foundation Conference, American Society of Civil Engineers, New York, NY. pp. 651-662 (1994).
Lee, G.F. and Jones-Lee, A., "Stormwater Runoff Management: The Need for a Different Approach," Water/Engineering & Management, 142:36-39 (1995).
Lee, G.F. and Jones-Lee, A., "Implementing Urban Stormwater Runoff Quality Management Regulations," Water/Engineering & Management, 142:38-41 (1995)
Lee, G.F. and Jones-Lee, A., "Issues in Managing Urban Stormwater Runoff Quality," Water/Engineering & Management, 142:51-53 (1995)
Lee, G.F. and Jones-Lee, A., "Storm Water Runoff Management: Are Real Water Quality Problems Being Addressed by Current Structural Best Management Practices? Part 1," Public Works, 125:53-57, 70-72 (1994). Part Two, 126:54-56 (1995).
Lee, G.F. and Jones-Lee, A., "Independent Applicability of Chemical and Biological Criteria/Standards and Effluent Toxicity Testing," The National Environmental Journal, 5(1):60-63, (1995), Part II, "An Alternative Approach," 5(2):66-67 (1995).
Lee, G.F and Jones-Lee, A., "Storm Water Runoff Quality Monitoring: Chemical Constituent vs. Water Quality, Part I, II," Public Works, Part I 147:50-53 (1996), Part II 147:42-45, 67 (1996).
Lee, G.F. and Jones-Lee, A., "Appropriate Use of Numeric Chemical Water Quality Criteria," Health and Ecological Risk Assessment, 1:5-11 (1995). Letter to the Editor, Supplemental Discussion, 2:233-234 (1996).
Lee, G.F. and Jones-Lee, A., "Automobile Brake Pad Copper: Is There a Real Water Quality Problem? An Example of an Inappropriate Approach for Developing a Stormwater Runoff Source Control BMP," Report of G. Fred Lee & Associates, El Macero, CA, 18pp, June (1996).
Lee, G.F. and Jones-Lee, A., "Aquatic Chemistry/Toxicology in Watershed-Based Water Quality Management Programs," Proc. Watershed '96 National conference on Watershed Management, Water Environment Federation, Alexandria, VA, pp. 1003-1006 (1996).
Lee, G.F. and Jones-Lee, A., "Results of Survey on Water Quality Problems Caused by Urban and Highway Stormwater Runoff," Runoff Reports, 4(5):3 (1996).
Lee, G.F. and Jones-Lee, A., "Evaluation Monitoring as an Alternative to Conventional Storm water Runoff Monitoring and BMP Development," SETAC News, 17(2):20-21 (1997)
Lee, G.F. and Jones-Lee, A., "Regulating Copper in San Francisco Bay: Importance of Appropriate Use if Aquatic Chemistry and Toxicology," Presented at the Fourth International Conference on the Biogeochemistry of Trace Elements, Berkeley, CA, June (1997e).
Lee, G.F. and Jones-Lee, A., "The Appropriateness of Using U.S. EPA Water Quality Criteria as Goals for Urban Area and Highway Stormwater Runoff Water Quality Management," Report of G. Fred Lee & Associates, El Macero, CA, March (1997).
Lee, G.F. and Jones-Lee, A., "Proposed Policy for Urban Area and Highway Stormwater Runoff Water Quality Management," Report of G. Fred Lee & Associates, El Macero, CA, February (1997).
Lee, G.F, Watson, R.A. and Taylor, S.M., "Evaluation Monitoring Program Baseline Implementation Project.," In: Technical Appendices Runoff Management Plan for the Eastern Transportation Corridor, Volume 2, Silverado Constructors, Irvine, CA, February (1997).
Lee, G.F. and Jones-Lee, A., "Development of a Storm water Runoff Water Quality Evaluation and Management Program for Hazardous Chemical Sites," To be presented at ASTM Third Symposium on Superfund Risk Assessment, San Diego, CA, January (1998).
Norberg-King, T.J. et al., "Toxicity Identification Evaluation: Characterization of Chronically Toxic Effluents, Phase I." EPA-600/6-91/005, U.S. Environment Protection Agency, Office of Research and Development, Environmental Research Laboratory, Duluth, MN, June (1991).
Rand, G.M., (Ed.). Fundamentals of Aquatic Toxicology: Effects, Environmental Fate, and Risk Assessment, 2nd edition Taylor and Francis, Bristol. PA (1995).
U.S. EPA, "Biological Criteria: National Program Guidance for Surface Waters," EPA-440/5-90-004, U.S. Environmental Protection Agency, Office of Water Regulations and Standards, Washington, D.C. (1990).
U.S. EPA, "Technical Support Document of Water Quality-Based Toxics Control," EPA/505/2-90-001, U.S. Environmental Protection Agency, Office of Water. Washington, D.C. (1991).
U.S. EPA, "Methods for Aquatic Toxicity Identification Evaluations, Phase I Toxicity Characterization Procedures, Second Edition," EPA/600/6-91/003, U.S. Environmental Protection Agency, Office of Research and Development, Washington, D.C., February (1991).
U.S. EPA, "Methods for Aquatic Toxicity Identification Evaluations: Phase II Toxicity Identification Procedures for Samples Exhibiting Acute and Chronic Toxicity," EPA/600/R-92/080, U.S. Environmental Protection Agency, Office of Research and Development, Cincinnati, OH, September (1993).
U.S. EPA, "Methods for Aquatic Toxicity Identification Evaluations: Phase III Toxicity Confirmation Procedures for Samples Exhibiting Acute and Chronic Toxicity," EPA/600/R-92/081, U.S. Environmental Protection Agency, Office of Research and Development, Cincinnati, OH, September (1993).
U.S. EPA, "Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Marine and Estuarine Organisms, Second Edition," EPA 600/4-90/003, U.S. Environmental Protection Agency, Environmental Monitoring and Support Laboratory, Cincinnati, OH (1994).
U.S. EPA, "Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Water to West Coast Marine and Estuarine Organisms, First Edition," EPA/600/R-95/136, U.S. Environmental Protection Agency, Environmental Research Laboratory, Cincinnati, OH (1995).
U.S. EPA, "Method 1669: Sampling Ambient Water for Trace Metals at EPA Water Quality Criteria Levels," EPA 821-R-95034, U.S. Environmental Protection Agency, Office of Water, Washington, D.C. (1995).
U.S. EPA, "Interim Permitting Approach for Water Quality-Based Effluent Limitations in Storm Water Permits," U.S. Environmental Protection Agency, Office of Water, Washington, D.C., August 1, (1996).
U.S. EPA, "Advance Notice of Proposed Rule Making," Water Quality Criteria and Standards Newsletter, EPA-823-N-95-006, U.S. Environmental Protection Agency, Office of Water, Washington, D.C., February (1996).