Reprinted from Transactions Northeast Fish and Wildlife Conference May 1972 p. 39-60
EUTROPHICATION
G. Fred Lee
Institute for Environmental Sciences
The University of Texas at Dallas
P.O. Box 688
Richardson, Texas 75080
ABSTRACT
The eutrophication-excessive fertilization of natural waters is becoming one of the most important causes of water quality deterioration. The cultural activities of man greatly accelerate the transport of phosphorus, nitrogen and other elements which may limit aquatic plant growth in natural waters. These nutrients stimulate the growth of floating or suspended algae, attached algae and macrophytes. Excessive growths of these aquatic plants result in a significant deterioration in water quality for the use of the water for domestic and industrial water supplies, irrigation and recreation.
The control of eutrophication is normally based on limiting aquatic plant nutrient input. Such control efforts must be directed toward the element which is currently limiting or can be made to limit algal growth in the body of water of concern. Domestic wastewaters represent potentially significant sources of nitrogen and phosphorus for the excessive fertilization of surface waters. Eutrophication control efforts are generally directed toward limiting the phosphorus content of domestic wastewaters by precipitation or co-precipitation treatment methods involving the use of aluminum or iron salts or lime. Other potentially significant sources of nitrogen and phosphorus include urban and rural stormwater drainage and atmospheric inputs. One of the most important sources of phosphorus in the upper Midwest is the manure spread on frozen soil from dairy farming and feedlot operations. The control of nitrogen and phosphorus from urban and rural diffuse sources is a much more difficult task and will require the expenditure of large amounts of funds if excessive fertilization of natural waters it to be minimized to the greatest possible extent.
The fertilization of natural waters may result in the production of excessive growths of algae and higher plants. These crops may cause a serious deterioration of water quantity. Today, the excessive fertilization (eutrophication) of natural waters is one of the most significant causes of water quality problems in North America. It is likely that as other sources of pollution are abated, the problems of excessive fertilization will assume a greater role in the deterioration of aquatic environmental quality. Eutrophication problems are not restricted to lakes; they also occur in rivers, impoundments, estuaries and other coastal waters.
This paper discusses the general aspects of the eutrophication of natural waters. Emphasis is placed on methods of measuring eutrophication, on the causes of eutrophication (particularly the sources of aquatic plant nutrients and the factors that influence aquatic plant production), on the effects of eutrophication on water quality and oil methods of controlling or reducing the rate of eutrophication. Additional information on these topics can be obtained in the following papers and reviews: Anonymous (1961), Edmonson (1968), Frey (1963), Hasler (1947), Hutchinson (1957, 1967), Jackson (1964, 1968), Lee and Fruh (1966), Machenthun (1962), Machenthun and Ingram (1967), Machenthun et al. 1964), National Academy of Science (1969), National Technical Advisory Committee (1968), Sawyer (1966), Stewart and Rohlich (1967), Veatch and Humphrys (1964) and Vollenweider (1968).
CLASSIFICATION OF LAKES
Lakes and other surface waters are frequently divided into one of two types, oligotrophic and eutrophic. Although the exact meaning of these two terms depends on the user, it is generally agreed that oligotrophic lakes are relatively unproductive and receive small amounts of aquatic plant nutrients, while eutrophic lakes are highly productive and experience high fluxes of aquatic plant nutrients.
The problem of defining these terms, oligotrophic and eutrophic, is related to the fact that some aquatic scientists use them to refer only to the flux of aquatic plant nutrients, while others use them to describe the amounts of plant and/or animal production. Still others use them to relate nutrient flux to water quality. In reality, with few exceptions, the various definitions all are essentially the same since high nutrient fluxes result in higher plant and animal production and decreased water quality.
Table I. General Characteristics Frequently Used to Classify Lakes
|
Type of lake |
||
|
Parameter |
Oligotrophic |
Eutrophic |
|
Aquatic plant production |
Low |
High |
|
Aquatic animal production |
Low |
High |
|
Aquatic plant nutrient flux |
Low |
High |
|
Oxygen in the hypolimnion |
Present |
Absent |
|
Depth |
Tend to be deeper |
Tend to be shallower |
|
Water quality for most domestic and industrial uses |
Good |
Poor |
|
Total salts or conductance |
Usually lower |
Sometimes higher |
|
Number of plant and animal species |
Many |
Fewer |
Table I summarizes the general characteristics of oligotrophic and eutrophic lakes. Oligotrophic lakes contain small amounts of organisms but many different species of aquatic plants and animals. Eutrophic lakes are generally thought to contain large numbers of aquatic plants and animals of a few species. Recent evidence seems to indicate that eutrophic lakes may contain as many different species as oligotrophic lakes. The difference between the two is primarily that in eutrophic lakes there tends to be one or two species that dominate the population. Normally, lakes that are sufficiently deep to develop a thermocline (two-layer system due to density differences as a result of the thermal structure) and show a partial or complete depletion of dissolved oxygen in the hypolimnion (bottom layer) are classified as eutrophic, while those that maintain the oxygen in the hypolimnion throughout the period of thermal stratification are oligotrophic. T his oxygen depletion in the bottom waters of a lake is dependent on amounts of aquatic plants that develop in the surface water and the morphology of the lake. For lakes with a given amount of aquatic plant production in the epilimnion (surface layer), those with a large hypolimnetic volume tend to have the oxygen concentration depleted to a lesser degree than those with a smal hypolimnetic volume. With few exceptions, the amount of aquatic plants produced in a lake is restricted to the surface waters since light penetration is usually restricted to epilimnetic waters.
In addition to differences in the total amounts of aquatic plant and animal production and the numbers of types (species) of each, the two types of lakes vary as to the type of organisms produced. Although no indicator species have been found that are only present in one type of lake, it is generally observed that eutrophic lakes have greater numbers of blue-green algae and rough fish such as carp. At times, statements appear in the popular press that a eutrophic lake, like Lake Erie, is dead. Actually, this is exactly opposite to the real situation in that eutrophic lakes suffer from problems due to excessive amounts of life of all forms. The fertilization of a body of water increases all forms of life from the planktonic (freely suspended) algae, attached algae and rooted higher plants that develop in nearshore water to the zooplanklon, benthic organisms and fish.
Oligotrophic lakes arc usually deeper than eutrophic lakes. However, this characteristic is highly variable and it is probably best to state that shallow lakes are frequently eutrophic and that as an oligotrophic lake fills due to deposition of sediments, it will become more eutrophic.
Another characteristic that is sometimes used to classify lakes is the total dissolved salt content or specific conductivity The use of this parameter is based on an empirical correlation between the general levels of plant and animal production and total dissolved salts. Frequently lakes with higher dissolved salts tend to be more productive. Beeton (1965) has presented as evidence for the eutrophication of the St. Lawrence Great Lakes an increase in the dissolved salt content of these waters. Chemical species that make up the bulk of the total salts in most lakes are calcium (Ca++), magnesium (Mg++), sodium (Na+), potassium (K+), sulfate (SO4=), bicarbonate (HCO3-) and chloride (Cl-). Sometimes nitrate (NO3-) concentrations are encountered in ground waters in amounts that contribute to the dissolved salts or specific conductance of the water. With the exception of nitrate, all of these species play minor roles in aquatic plant production of a lake. i.e., their concentrations are in sufficient excess so that they do not limit the plant production. Therefore, high total salts in a lake usually mean that the lake also has a high content of aquatic plant nutrients.
In the case of the St. Lawrence Great Lakes, the increase in dissolved salts with time observed by Beeton was the result of a greater degree of cultural activity in the lakes' drainage basins and thus an increased flux of many of the common cations and anions normally found in lakes. As will be shown later, associated with the increase in cultural activity, a higher nitrogen and phosphorus flux will also occur. Although the total amounts of nitrogen, phosphorus and other aquatic plant nutrients are insufficient to be determined as a part of the dissolved salts or specific conductivity, they are sufficient to cause excessive growths of algae and other aquatic plants in Lake Erie and parts of the Great Lakes. Even though these salts probably do not significantly contribute to the nutrients for aquatic plants, their buildup in the Great Lakes is of major concern and will require their removal from some waste waters in the foreseeable future.
Limnologists use many other terms to classify lakes, such as dystrophic, mesotrophic, hypereutrophic, etc. Some of these terms bear little or no relationship to the nutrient flux, while others do (i.e., they are subdivisions of the oligotrophic-eutrophic classification). For the purposes of this discussion, oligotrophic and eutrophic classification is adequate to describe the relationship between nutrient flux and water quality problems.
CLASSIFICATION OF EUTROPHICATION
Once a lake has been found to be eutrophic, it is further classified as to the process by which the eutrophication took place. Some lakes and other surface waters are naturally eutrophic, i. e., they receive sufficient aquatic plant nutrients from natural sources to produce excessive crops of algae and macrophytes. Many references in the literature state that lakes start as infertile bodies of water, with time accumulate nutrients, become more fertile, eventually fill with sediments and become a marsh and then dry land (Hasler, 1947). However, studies on the chemical characteristics and organism remains in lake sediment cores indicate that lakes do not become more fertile with time. In fact, it is likely that lakes are highly fertile immediately after formation and may lose this fertility as the easily leachable nutrients are removed from the watershed.
Regardless of whether these lakes become more or less fertile with time, natural eutrophication accelerates when a lake becomes shallow enough due to sediment accumulation so that it loses its permanent stratification. At this point, nutrients are no longer trapped below the thermocline, and the lake becomes more fertile since its nutrients are more rapidly recycled from its sediments. A final major transformation in the natural eutrophication of a lake is the point where the lake is sufficiently shallow so that macrophytes occupy a major part of the lake basin. At this point in time, the lake starts to fill at a markedly different rate due to partial degradation of higher aquatic plant remains. Whether the natural eutrophication of lakes follows the sigmoid growth curve or proceeds in a stepwise manner, as proposed here, will await further research (see Figure 1).
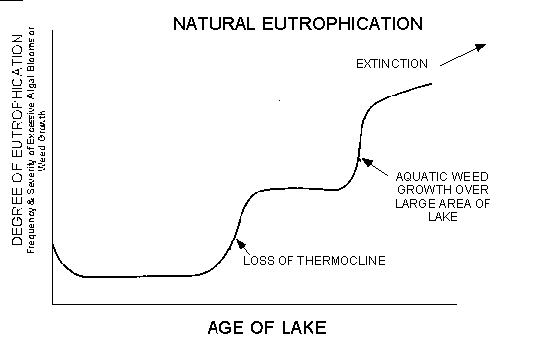
It might be argued that the shape of the natural eutrophication curve should have a step in it to correspond to the time that the hypolimnetic waters of the lake become anoxic to any significant degree. While it is clear that deoxygenation of the hypolimnion does lead to and cause large releases of many aquatic plant nutrients such as nitrogen and phosphorus, it is doubtful that this release plays an important role in fertilizing the lake during the critical use period of the summer. The thermocline appears to be a very effective barrier to the transport of nutrients from deeper sediments of lakes to the overlying water. Fall overturn or mixing in the lake does result in the mixing of high-nutrient hypolimnetic waters with the epilimnetic waters. Often this will result in an algal bloom. Normally, by this time of year the water has cooled sufficiently so that the algae that cause the severe summer blooms (blue-greens) do not develop. Although the fall blooms can cause serious water quality problems from a water supply use point of view, these blooms rarely cause serious recreational use problems because the waters generally are not used as intensively in the fall as in the summer.
The high nutrient level that develops at the beginning of fall overturn rarely persists for any period of time since the fall algal blooms tend to carry the nutrients to the sediments and, more importantly, the ferrous iron that was present in the hypolimnion is oxidized to ferric iron by dissolved oxygen and precipitates as ferric hydroxide, carrying with it coprecipitated and occluded phosphorus to the sediments. Based on the current degree of understanding of the aqueous environmental chemistry of phosphorus, it is unlikely that the aquatic plant nutrients brought to the surface waters at this time play a major role in increasing the degree of eutrophication of a lake, and therefore no provision has been made to include a step in this natural eutrophication diagram to reflect the time in a lake's history that the hypolimnion becomes anoxic to a significant degree. It should be pointed out that research in systems models of the water chemistry of plant nutrients will likely produce changes in the shape of this diagram.
While these lakes become naturally eutrophic in the absence of man, other lakes are culturally eutrophic, i. e., they receive aquatic plant nutrients from artificial sources or, more specifically, as a result of man's presence. The activities of man in a lake's watershed can bring about the cultural eutrophication of a lake in a period of a few years, whereas natural eutrophication is a very slow process and is estimated as occurring over hundreds to thousands of years. Because of this marked difference in the normal relative rates of cultural versus natural eutrophication, it is likely that any measurable change in the tropic state of a lake in the past 50 years is due to cultural rather than natural causes.
CAUSES OF EUTROPHICATION
A great variety of influences contribute to the eutrophication of a water. These are discussed as a background to considering the effects of eutrophication and means of reducing them.
FACTORS THAT INFLUENCE AQUATIC PLANT PRODUCTION
The productivity of a given body of water is dependent on solar radiation, temperature (to some degree) morphology and the concentration of aquatic plant nutrients present in water and/or available to organisms.
Aquatic plant production is apparently not inhibited by cold temperatures since excessive algal populations are found in waters near the freezing point. Frequently, very high concentrations of blue-green algae will be found in temperate lakes growing under a meter or more of ice, although some of the more objectionable forms of algae such as the blue-greens are generally considered warm-water organisms.
The discharge of waste heat from large electric generation stations such as nuclear power plants is often alleged to cause an increased eutrophication of a lake. While it is true that blue-green algae tend to be more prevalent in warm waters, there is no reason to believe that the total biomass of the organism would change significantly as a result of heating the water a few degrees above ambient. Normally, the biomass of a certain type of algae during a season is dependent on availability of aquatic plant nutrients rather than on factors such as temperature. There is serious doubt that the highly localized heating of water resulting from large nuclear-power electric generating stations located on large lakes should have an effect on the over-all degree of eutrophication of the take as a whole. It is possible that, where suitable substrates for the attached algae exist in the region of the discharge plume of the electric generating station, these forms might develop a little earlier each spring and persist slightly longer each fall. However, it is doubtful that the total amounts of these organisms would be increased during the critical recreational use during summer months.
The amounts of planktonic algae would not be expected to increase due to discharge of waste heat to large lakes since their rate of growth is normally much slower than the time they would be exposed to elevated temperatures in most electric generating station thermal plumes.
Light does limit the aquatic plant production in waters which have large amounts of suspended solids that increase turbidity or have high color. Normally, lakes of this type are very shallow and are subject to strong mixing by wind or animals such as fish or receive large amounts of marsh or swamp drainage.
The role of morphology of a lake in deter mining its productivity is discussed under a subsequent topic.
Of factors thus far considered, if light, temperature, basin morphology and residence time of water are "normal," a sufficiently high flux of aquatic plant nutrients can create eutrophication problems. Even if these other factors are not "normal," i. e., they are operating to increase aquatic plant production. This production is still most frequently controlled by the flux of chemical compounds that influence algal growth. Although the nutritional requirements of algae and higher aquatic plants are poorly understood, it is generally agreed that they all have large requirements for carbon (C), nitrogen (N), hydrogen (H), oxygen (O), and phosphorus (P) and need lesser amounts of many different trace elements (Anonymous, 1967; Fogg, 1965; Goldman, 1966; Irwin, 1962; Machenthun, 1965; Machenthun and Ingram, 1966; Middlebrooks et al., 1969; Vollenweider, 1969). The nutrient requirements of aquatic plants are much the same as those of terrestrial plants except for the need for potassium by terrestrial plants. The fixation of potassium by clay minerals causes a deficiency in soils, while in water there is generally sufficient potassium to meet the needs of the aquatic plants. Aquatic plants are different in one important respect from some terrestrial plants in that they can use either NO3- or NH4+ with about equal facility, while some terrestrial plants are reported to use only NO3-. Some algae will take up NH4+ in preference to NO3-. Some of the blue-green algae also have the ability to use N2 gas. Since the dissolved N2 content of water is always high, the process of N2 fixation gives this group of algae the ability to grow at very low NO3- and NH4+ concentrations. Orthophosphate (PO4) is the form of phosphorus that is readily available for algal growth although it is possible that other forms such as P-O-P and organic phosphorus may also be available to algae. The role of these compounds in the growth of algae is discussed under a subsequent topic.
TYPES OF AQUATIC PLANT NUTRIENTS
The aquatic plant nutrients often considered to be the most significant ones limiting the growth of aquatic plants in surface waters are orthophosphate, nitrate, nitrite and ammonia. Other species of phosphorus and nitrogen, such as the organic forms, can and do contribute to the fertility of the water, either directly through uptake by the organisms or indirectly through their conversion to the other previously mentioned forms by abiotic and biotic chemical reactions.
There are some who feel that carbon should be listed as one of the critical elements in terms of the fertilization of natural waters. However, evidence to date seems to indicate that carbon (in the form, CO2) becomes limiting only in the presence of excessive or massive blooms of algae and that under these conditions the degree of eutrophication is exceedingly high. At that time, water quality has deteriorated to such a degree that further plant production is of limited consequence.
With regard to the trace elements, it is known that some are essential for growth of plants; for example, the diatoms need silica for growth. However, even with the diatoms, silica is generally not a limiting element, but is in excess 6f what is needed for the production of large numbers of these organisms.
Probably the next most frequently mentioned element that could possibly limit or control the populations of algae in lakes is iron. Iron is present in the epilimnion of lakes in the ferric form; in this state of oxidation, it is extremely insoluble as a hydrous oxide and is rapidly precipitated and transmitted to the sediments of the lake. Since some investigators have found an apparent iron deficiency in some waters, iron may indeed be an element limiting plant growth.
Other elements that are mentioned as essential for growth of algae and other aquatic plants include sulfur, potassium, magnesium, calcium, boron, zinc, copper, molybdenum, manganese, cobalt, sodium and chlorine. Regarding these elements and various organic components of water such as vitamins, hormones and chelating agents, the question must hr asked do they control (i.e., limit) algal growth or are they simply essential and in sufficient concentrations to provide for excessive crops of aquatic plants?
It is fair to state that nitrogen and phosphorus are probably the key elements in controlling aquatic plant growths and that the other elements, compounds and components of water may be important under certain conditions in certain types of waters. Further research will be necessary to fully delineate their roles in the eutrophication of natural waters.
CONCENTRATIONS AND SOURCES OF NITROGEN AND PHOSPHORUS
Since nitrogen and phosphorus appear to be the most important plant nutrients, the question may be asked: what are the critical concentrations of these two nutrients in most natural waters? This is a topic of great concern today in terms of attempting to manage the nutrient input to lakes. If the nutrient input can be limited so that the total concentrations in the lake are less than the critical concentrations, beyond which eutrophication will occur, then it would be possible to control the degree of eutrophication of the water. Although few studies have been made, reports by Sawyer et al. (1945) and Vollenweider (1961) pertaining to Wisconsin lakes and Swiss lakes, respectively, indicate that when the inorganic nitrogen content (ammonia plus nitrate nitrogen) is equal to or greater than 0.3 milligram per liter of nitrogen and the orthophosphate content is equal to or greater than 0.01 milligram per liter of phosphorus, then the lake is likely to have excessive crops of algae and other aquatic plants. These numbers represent, to some degree, the best estimates available today of the critical concentrations of these elements.
Unfortunately, the relationship between these concentrations and aquatic growth is a complicated one and the use of these concentrations to predict or prevent the eutrophication of a given body of water is difficult for the following reasons:
1. The productivity of a given body of water, as pointed out previously, depends on other factors in addition to the concentrations of nutrients. For example, the species of algae and other aquatic plants are determined not only by the amounts of, but also by the ratios between, the various elements.
2. Even if productivity were controlled only by nutrient concentrations, it is not certain at the present time whether nitrogen and/or phosphorus is (are) the controlling element(s). The evidence obtained in the past few years seems to show that nitrogen controls a certain population at certain times during the year while phosphorus in the same lakes may be controlling another population at another time.
3. The critical concentrations proposed by Sawyer and Vollenweider are based on excessive growths of algae as the quality criteria. While these concentrations might be used to predict one kind of eutrophication (caused by excessive plant growths), they overlook those water quality problems produced by much lower concentrations of nutrients. Specific forms of algae, for example, are known to cause taste and odors in water supplies or to produce clogging in sand filters in water treatment plants.
4. The stoichiometric concentrations of some of the primary elements in algae vary within fairly wide limits. Both fresh-water and marine algae contain, on the average, 106 carbon atoms for each 1 phosphorus and 16 nitrogen atoms. Algae, though, have the ability to store what is termed surplus phosphorus which ranges from five to approaching 10 times the minimum requirements for the particular cell. The composition of algae is therefore highly variable and dependent on the composition of water.
5. Furthermore, some algae have specific phosphorus and/or nitrogen requirements which are markedly different from those of other algae. Phosphorus levels which are greatly below the critical concentrations found by Sawyer may be sufficient to support the growth of some algae, yet Sawyer's critical concentrations of phosphorus might actually be toxic to these same algae.
6. Finally, attempts have been made to use the critical concentrations to "limit" the phosphorus content of a given body of water. Such attempts, recently tried by both federal and state governments, do not, however, take into account the complex aqueous environmental chemistry of phosphorus. Trying to control the nutrient input for a given lake, so that its phosphorus levels are kept below Sawyer's critical concentrations, ignores the fact that the sediments of a lake act as a sink and source for many of the nutrient elements. Thus, input control may not limit the actual phosphorus concentrations to a lake because of the rate at which this element is transferred from the sediments to the water. The role of sediments in supplying nutrients to the overlying waters is discussed under a subsequent topic.
References cited under "Factors That Influence Aquatic Plant Production" should be consulted for additional information on the nutritional requirements of algae and other aquatic plants. With this background, it is appropriate to examine the various sources of nitrogen and phosphorus compounds in an attempt to gain some insight into the potential causes of water-quality problems doe to excessive fertilization. Additional information may be obtained from references: Anonymous (1967, 1969), Lee (1966) and Weibel et al. (1964).
DOMESTIC WASTE WATERS
One of the foremost sources of nitrogen and phosphorus in natural waters is domestic waste waters. Domestic waste waters often contain tens of milligrams per liter of nitrogen and from 5 to 20 milligrams per liter of phosphorus . The concentrations of nitrogen and phosphorus in domestic waste waters are highly variable and are dependent on the time of day and day of the week. If these concentrations are compared to the critical concentrations, it is readily apparent that domestic waste waters must be diluted manyfold in order to reduce the fertilizing potential below those levels cited by Sawyer as critical concentrations.
Of major concern today are the sources of phosphorus in domestic waste waters. Several studies have shown that approximately 50 per cent of the phosphorus present in domestic waste waters is derived from the phosphorus that is used in various cleaning compounds such as detergents. Many detergent formulations contain condensed phosphates which have been shown to hydrolyze to orthophosphate during domestic waste water treatment or in natural waters. Some attempts are being made today to try to limit the amounts of phosphates used in detergents to reduce the fertilizing potential of domestic waste waters.
A crude estimate of the amounts of nitrogen and phosphorus contributed by domestic waste waters can be made in terms of a population equivalent. Various studies have shown that 2 to 3 pounds per person per year of phosphorus and 7 pounds per person per year of nitrogen are contributed to typical domestic waste waters. These figures, then, can be used to obtain crude estimates of the potential significance of a given waste water in terms of its nitrogen and phosphorus contribution to a watercourse.
Eutrophication problems may also result from sludge disposal associated with waste water treatment. Sludge and other solids are usually placed on the ground or in a pit, and garbage is frequently disposed of on low lands which have high water tables. Nutrients from these areas can be carried into the ground water and, during periods of heavy rains, into surface waters via runoff. High nitrogen and phosphorus content waters are associated with both domestic wastewater sludge disposal and garbage and refuse disposal areas.
The individual household septic tank disposal system may also contribute nutrients to surface waters. In general, nitrogen is readily transported in ground waters, and, therefore, a significant part of the nitrogen going to septic tank systems will be transported to the ground water if it is oxidized to nitrate and does not become denitrified. Phosphate is usually strongly sorbed by aquifer materials except in sandy areas. Quartz and other sands that have low iron, carbonate. aluminum, clay mineral and organic content will readily transport phosphate in ground waters. In nonsandy soils, septic tanks frequently fail in a short period of time due to the plugging of the tile field. When this occurs, surface discharge results and nutrients are transported via overland flow to the nearby watercourse.
INDUSTRIAL WASTE WATERS
Industrial waste waters can have very large or very small amounts of aquatic plant nutrients. For example, some waste waters which cause serious; water-quality problems, such as waste water from pulp and paper plants, obtain relatively small amounts of nitrogen and phosphorus, but large amounts of carbon. However, waste waters, such as those associated with phosphate mining, do contribute large amounts of phosphorus to the water. The nutrient content of industrial waste waters may or may not be serious and varies front industry to industry.
URBAN DRAINAGE
Runoff from urban areas is beginning to be recognized as an important source of aquatic plant nutrients. Frequently, stormwater runoff contains a few milligrams per liter of nitrogen and phosphorus. Weibel et al.(1964) found that in Cincinnati approximately 9 pounds per acre per year of nitrogen and 0.8 pound per acre per year of soluble phosphorus are contributed in stormwater runoff. At this time, little information is available on the sources of these high concentrations in streets, although fertilizers used on lawns, normal dust fall, leaves, waste materials from domestic pets, gasoline automobile engines and other, combustion processes must be considered as possible sources. Further research, though, will be necessary to delineate the relative significance of these sources.
AGRICULTURAL DRAINAGE
Because of the fact that large quantities of fertilizers are often used on various crops, agricultural drainage is often thought to be a significant source of nutrients for surface waters. Although under proper soil, water and fertilizer management, it is possible to control the amounts of nitrogen and phosphorus derived from fertilizers applied to croplands, misapplication of these materials can result in large amounts being carried to surface waters. Studies of farmlands located near Madison, Wisconsin, have shown that the nutrient content of farmland drainage can range from that essentially associated with natural sources, with concentrations on the order of 0.03 and 0.003 pound per acre per year of nitrogen and phosphorus, respectively, to values many times this amount where applied fertilizers are used in great excess of their needs or where conditions tend to promote their runoff to the nearby surface waters.
One of the most significant sources of nutrients in the agricultural drainage for the area near Madison, Wisconsin, is the manure derived from dairy farm operations. In this part of the country, dairy farmers maintain approximately 100 cows per square mile, each cow producing 15 tons of manure per year. When this manure is spread on the land during late spring, summer and early fall, it is reasonable to expect that the nutrients present in the manure will be carried into the soil and, in general, will become associated with the crops that develop. However, during the winter period the manure spread on the land cannot leach its nutrients to the frozen soil. At the time of spring thaw and rain, the snow melt and rainwater combine in a heavy overland flow, carrying with it the nutrients from the manure spread on frozen land.
In general, the manure problem associated with dairy operations is small compared to the manure problems found on animal feedlots where the density of animals is much higher. Feedlots often have animal densities approaching 50 to 100 animals per acre, and some lots contain as many as 100,000 animals. Drainage from these large feedlots requires treatment similar to the treatment needed for domestic waste waters.
In the western United States, the irrigation return waters have high concentrations of nitrogen which may cause eutrophication problems. Some irrigation return waters have been found to contain concentrations of nitrate approaching 50 milligrams per liter of nitrogen. These waters require treatment to remove the very high concentrations of nitrogen.
NATURAL SOURCES
Many lakes are naturally eutrophic, i.e., they receive large enough quantities of nitrogen, phosphorus and other plant nutrients to produce excessive crops of aquatic plants. Under these conditions, the watershed of the lake serves as the nutrient source and will supply the lake with large amounts of nitrogen and phosphorus. Some soils, such as many of those located in the Midwest, are highly fertile and contain large amounts of nitrogen and phosphorus, with the result that small lakes which receive drainage from large land areas are often naturally eutrophic.
LAKE SEDIMENTS
Sediments of a lake often contain thousands of parts per million (millograms per kilogram dry weight) of phosphorus and nitrogen. If the sediments are mixed with the water and contain leachable nutrients, potentially very small amounts of sediments could fertilize large quantities of water. Fortunately, however, most of the nitrogen and phosphorus present in lake sediments are bound in refractory (nonleachable) forms.
Recent studies have shown that some lake sediments can act as phosphate buffers. If the phosphorus concentration in the overlying waters is decreased, the sediments will contribute phosphorus to the overlying waters to make up for this decrease. Sediments will also contribute nitrogen to overlying waters, although nitrogen appears. to be controlled more by the aerobic and anaerobic conditions (Lee, 1970). Further studies on the role of lake sediments as a source of nitrogen and phosphorus for lakes are urgently needed. Recently-completed studies on the rates of recovery of lakes upon a nutrient to phosphate input reduction have shown that the sediments of the lakes do not significantly affect the lake's recovery rate.
ATMOSPHERIC SOURCES
Precipitation in the form of rainfall and snowfall is a significant source of nitrogen in some parts of the world. In the Midwest, typical rainfall contains on the order of 1 milligram per liter of nitrate nitrogen. When this is compared to the normal 0.3 milligram per liter often cited as the critical concentration of nitrogen, rainfall rather than diluting the nutrients in a lake is actually contributing to them. Phosphorus is fairly low in most rainfall, although some recent data indicate that lakes in some parts of the United States receive significant amounts of phosphorus from atmospheric sources in the form of precipitation and dust fall.
GROUND WATER
Ground water rarely contains significant concentrations of phosphorus since phosphorus species tend to be strongly sorbed on aquifer materials. But ground waters can contain large concentrations of nitrate nitrogen since nitrate is very poorly sorbed by most aquifer materials. It is not uncommon to find that nitrate nitrogen is present in ground waters at 1 milligram per liter of nitrogen. In some situations, this concentration can reach 40 to 50 milligrams per liter. Ammonia nitrogen and various phosphate species tend to he sorbed in most clay soils. However, in sandy soils, such as those frequently encountered around glacial lakes, it may be found that the sorption capacity of sand is exceedingly small with the result that septic tank disposal systems located adjacent to a lake in a sandy soil rarely have problems of plugging. However, these systems also readily transmit the nutrients from the household to the nearby watercourse via ground water.
A special but frequent cause of ground contamination is sanitary landfills and other solid-waste, garbage, refuse and sludge disposal systems. Community waste-solid materials and wastewater sludges are deposited on lowlying land, frequently wetland areas. Recent studies have shown that serious ground water contamination problems have resulted from such practices. Other solid-waste and wastewater-sludge disposal systems must be initiated in order to avoid ground water contamination from various types of aquatic plant nutrients.
MARSHES
In general, marshy areas contain large populations of aquatic plants. It is reasonable to expect that these areas would act as nutrient traps and remove nutrients from inflowing waters during the growing season. However, during the winter and spring period in cold climates, marshes discharge large quantities of nitrogen and phosphorus, and in so doing probably act on a short-term basis to delay until times of high spring flow the outflow of nutrients. Therefore, it is reasonable to question whether or not a marsh is a nutrient trap over an annual cycle.
The drainage and conversion of marshes into either urban or agricultural areas can result in a significant release of nitrogen species, particularly nitrate. The marsh environment is typically an anaerobic one in which the nitrogen is present in the form of organic nitrogen and ammonia, both of which tend to remain associated with soil particles and plant debris within the marsh. However, draining a marsh converts it from an anaerobic to an aerobic environment which promotes biochemical conversion of organic nitrogen to ammonia and the conversion of ammonia to nitrate. Nitrate is highly soluble in water, poorly sorbed by soil particles and rapidly transported from the marsh systems The drainage of a marsh is likely to result in a significant increase in the nitrogen and phosphorus content in the effluent water.
NITROGEN FIXATION
Nitrogen fixation is restricted in natural waters to a few bacteria and a few forms of algae, particularly certain members of the blue-green algae. These organisms can convert nitrogen gas to ammonia and organic nitrogen. It is difficult at the present time to estimate the significance of nitrogen fixation. Certain forms of algae, such as Aphanizomeon and Anabaena are frequently associated wills highly eutrophic waters and are nitrogen fixers. On the other hand, Mycrosystis is also associated with highly eutrophic waters and it is not a nitrogen fixer. It is possible that nitrogen fixation occurs at such a rate as to not significantly affect the over-all nitrogen budget of a given body of water.
MISCELLANEOUS SOURCES
Among the miscellaneous sources of nutrients to lakes are ducks, geese and other organisms. On the one hand, wild duck populations probably do not add significant amounts of nutrients to the water. Since the ducks feed primarily in the water, they simply increase the rate of recycling of nutrients within the water. On the other hand, domestic duck populations such as those in zoos or in parks can contribute nutrients to the water in direct proportion to the amount of feeding by individuals that takes place. A similar problem exists with maintaining large herds of deer and other animals where these animals are fed near a lake shore. Wild geese feed primarily on land and, therefore, can increase the flux of nutrients from the land to the water.
Based on the foregoing discussion, aquatic plant nutrients are derived from many different sources. It is clear that for any given lake a detailed study of each of these potential sources must be made. A study of the nutrient sources for Lake Mendota (in Madison, Wisconsin) was made several years ago (Table 2). Examination of this table shows that the phosphorus is primarily derived from cultural sources, i.e., the waste waters or activities of man. Nitrogen for this particular lake, however, is derived from non-cultural sources such as rainfall, ground water, etc.
Table 2. Estimated Nutrient Sources of Lake Mendota*
|
|
Annual contribution |
Estimated contribution |
||
|
Nitrogen |
Phosphorus |
Nitrogen |
Phosphorus |
|
|
Municipal and industrial waste water |
47,000 |
17,000 |
10 |
36 |
|
Urban runoff |
30,300 |
8,100 |
6 |
17 |
|
Rural runoff |
52,000 |
20,000 |
11 |
42 |
|
Precipitation on lake surface |
97,000 |
140-7,6000 |
20 |
2 � |
|
Ground water |
250,000 |
600 |
52 |
2 |
|
Nitrogen fixation |
2,000 |
.. |
0.4 |
.. |
|
Marsh drainage |
? |
? |
.. |
.. |
|
Total |
478,300 |
47,000 � |
XXX |
XXX |
If other lakes had similar sources it would be logical to proceed with an attempt to control phosphorus inputs, since it would be extremely difficult, if not impossible, to control nitrogen. It may, however, not be possible to keep the elements below critical levels in any type of control program because of the wide variety of sources and particularly since some of these sources, such as stormwater runoff, are not amiable to control at the present time.
MEASUREMENT OF EUTROPHICATION
At the present time, there is no one single analytical tool to measure the degree of eutrophication of a given body of water. Instead, most experts feel the best approach is to measure many different parameters and to synthesize the results into a general pattern which will give an over-all, somewhat average degree of eutrophication for the water. This situation is likely to change within a few years since it may be possible, through the use of systems models, to develop numerical scales for comparing the eutrophication of one lake with that of another. However, even this approach will require large numbers of different parameters to be measured in order to assess the current degree of eutrophication. Fruh et al. (1966) have reviewed this subject. Many of the parameters that should be measured are mentioned under the following topics.
STANDING CROP OF ALGAE AND AQUATIC PLANTS
Since the problems of eutrophication are caused by excessive numbers of planktonic and attached algae and higher aquatic plants, it is logical to use the total numbers of these organisms present as an estimate of the current degree of the eutrophication of the water. Unfortunately, this is extremely difficult to do since it requires a team of scientists to collect the samples and enumerate and identify the organisms present. In order to circumvent this difficulty in obtaining this kind of information, numerous other methods have been proposed for assessing the total biomass of planktonic algae.
AMOUNT OF SUSPENDED SOLIDS
For example, the suspended solids in the water are sometimes used as a measure of the algal populations. The problem with suspended solids is that the majority in most waters are nonliving. In some lakes, there are roughly 10 times more nonliving than living particles of organic matter present in tile sample.
VOLUME OF ALGAE
Others have proposed that the volume of algae be used, i.e., the amount that would be obtained in a graduated centrifuge tube. Under extreme conditions of eutrophication this can give some estimate of the total numbers. Again, though, the problem of living versus nonliving matter must be considered.
AMOUNT OF CHLOROPHYLL
One of the most frequently used methods is one employing the extraction of chlorophyll from the sample. Chlorophyll is present in all plants in sufficient amounts that it can be extracted with various solvents and estimated by means of a spectrophotometer. The problem with chlorophyll is that it works reasonably well for relatively pure cultures of one type of organism. However, with mixed populations of organisms, particularly where there is one type of organism one week and another the next week, it is found that the correlation between the chlorophyll content and the total numbers of mass of algae in the water is frequently is very poor.
NUMBER OF ALGAL BLOOMS
In advanced stages of eutrophication, one of the somewhat subjective types of measurements that could be used to assess the degree of eutrophication is the number of objectionable blooms of algae. Bloom is a term used by aquatic scientists to mean a large number of a certain type of organism. Although there is no general agreement on the exact number of organisms that constitute a bloom, frequently 0.5 to 1 million cells per liter is used. A bloom of planktonic algae usually lasts in the order of a week or so during the summer period in a moderately eutrophic lake. Many lakes have half a dozen or so of these blooms per summer where the numbers of algae fluctuate widely from week to week. If some estimate of the frequency and severity of obnoxious blooms were recorded, it would be an important measure of the degree of eutrophication.
PHOTOSYNTHESIS
Many investigators have proposed that a measure of the photosynthesis of the organisms present in the water be used as a criterion for determining the degree of eutrophication of a body of water. Here, the problem is primarily one of correlating the activity as measured by photosynthesis with water quality problems. Some groups of organisms present in lakes could be photosynthesizing at a high rate, yet not cause significant water quality problems.
PRODUCTIVITY INDEX
Probably one if the better approaches to this is the combination of standing crop and primary production measured by photosynthesis. The productivity index proposed by Strickland and Parsons (1960) in which the primary production is divided by the biomass, might give a better correlation between the degree of eutrophication and its effect on man.
HYPOLIMNETIC OXYGEN CONCENTRATIONS
One of the more frequently-used parameters for assessing the eutrophication of lakes is the oxygen concentration in the hypolimnion. Hypolimnetic oxygen depletion in lakes that are sufficiently deep to stratify is probably the most widely used index for separating oligotrophic from eutrophic lakes. However, there are many problems with this index. The rate of depletion of oxygen from the hypolimnion of a lake depends to a significant degree on the lake's morphology, e. g., its area-to-volume ratio. Lakes that have large hypolimnetic volumes compared with their surface area will lose their oxygen at a significantly lower rate than lakes that have large surface areas and small hypolimnetic volumes. One of the complicating factors is the fact that in many lakes the most significant source of oxygen demand is the accumulated reduced iron, manganese and sulfide species in the lake sediments. Often eutrophic lakes contain thousands of parts per million of iron and sulfur species on a dry weight basis. These materials will react rapidly with dissolved oxygen. The lakes which are not stratified generally have a thin layer of oxidized iron hydroxide on the surface of the sediments. However, once the lake becomes stratified and renewal of oxygen from the atmosphere can no longer take place, this layer of oxidized iron oxide will be reduced, and in turnit is generally found that the oxygen in the hypolimnion is fairly rapidly depleted by abiotic chemical reactions. This statement should not be interpreted to mean that the primary production in the surface waters of a lake does nut control the hypolimnetic oxygen depletion; it does, particularly in oligotrophic and moderately eutrophic lakes. However, at some point in the eutrophication of a lake, there will be significant accumulation of reduced iron and sulfur species which can exert the major oxygen demand during periods of thermal stratification. Under these conditions the epilimnetic production of aquatic plants may change significantly through the years, yet the rate of depletion of the hypolimnetic oxygen will not change since it is controlled to some extent by abiotic reactions.
LIGHT PENETRATION
Light penetration is probably the most widely used measure of eutrophication. Although sometimes criticized as being an extremely crude instrument, the Seechi disk, which consists of a plate about a foot or so in diameter painted in black and white quadrants attached to a metered rope or line, is an important tool in limnological investigations involving eutrophication. One of the most significant effects of eutrophication on water quality is a decreased clarity of water. This decreased clarity of water results from light scattering from the microscophic plants that are produced in the water. The depth at which a Secehi disk can be dropped and still be visible is an estimate of the amount of phytoplankton present in the water. Although this technique involves many problems, it is an important one in that it is inexpensive and can he readily performed by almost any individual. In almost every case where it has been used, it is clearly shown that, as a lake becomes more eutrophic, the depth at which a Secchi disk can be seen decreases significantly.
AQUATIC PLANT NUTRIENT CONTENT
Another parameter that can he used to assess the degree of eutrophication of waters is the aquatic plant nutrient content. Although it is difficult to establish meaningful, critical concentrations that are applicable to many different waters, the nitrogen and phosphorus concentrations determined by Sawyer are often cited as being the critical levels that separate oligotrophic from eutrophic waters. These critical concentrations are often based on the excessive growths of aquatic plants that produce aesthetically unpleasing water. As has been pointed out under an earlier topic, it is quite possible that concentrations considerably less than these amounts could cause significant deterioration in water quality. particularly with respect to the use of the water for domestic water supplies. The use of these critical concentrations to divide oligotrophic from eutrophic lakes must be viewed with caution.
PLANT REGRESSION
Another parameter that could be used to estimate the degree of eutrophication of a lake is the regression of higher plants in the water. Here. we are concerned with the question of what is the maximum depth to which a rooted aquatic plant can grow in a given lake. This maximum depth is determined to a significant degree by light penetration. In some lakes it has been found that as the lake becomes more eutrophic the maximum depth at which higher plants can grow decreases. For example, plants may grow at 20 to 30 feet of water during the more oligotrophic stages of a lake, while in the eutrophic stages they may be restricted to depths of 5 to 10 feet. This is because the phytoplankton develop in sufficient numbers each year to block off the light to such an extent that plants which would normally grow in deeper waters in more oligotrophic conditions simply cannot develop because they do not receive sufficient light.
SEDIMENT COMPOSITION
A possible means of assessing the current degree of eutrophication of a lake is to measure the composition of its sediments. Sediment provide a geological record not only of the events that have occurred within a lake, but also of the events that have occurred within a watershed. It is extremely difficult at the present time to determine the sources of materials in the sediment. There is ample evidence that the carbon present in lake sediments, particularly the organic carbon, is derived primarily from the watershed; yet, it is reasonable to expect that, as a lake becomes more eutrophic, the total amount of organic carbon deposited in the sediments increases. It is also expected that as a lake becomes more eutrophic greater amounts of the phosphorus, nitrogen and other aquatic plant nutrients would be deposited in the sediments. Yet, at the present time, work in this area has not clearly demonstrated a relationship between the chemical composition of the sediments and the degree of eutrophication.
A more fruitful approach is to examine the sediments for specific types of organisms, particularly those with parts which are well preserved in the sediments. Probably one of the best groups for this purpose is the diatoms. The diatoms are a group of algae which have siliceous shells, many of which are well preserved in the sediments. By determining the number and types of such shells in sediments, it is possible to gain some inference about the rate of eutrophication of a given body of water.
In summary, there is no single parameter that can be used to estimate the current degree or rate of eutrophication of a lake or to compare the degree of eutrophication between lakes. Many different parameters must be used and each one must be weighed according to the general characteristics of the lake.
EFFECTS OF EUTROPHICATION
The eutrophication of a water may have a significant effect on domestic, industrial, recreational and agricultural uses of the water. Typical effects in each of these areas are discussed here.
DOMESTIC AND INDUSTRIAL USES
The presence of excessive amounts of nitrogen and phosphorus compounds can have a direct effect on water quality when ammonia exerts an increased chlorine demand and when the various phosphate species, particularly the condensed phosphates, affect the coagulation of particulates in the water with iron and aluminum salts. It is also possible that the nitrate content of the water could be increased significantly to cause problems of methemoglobinemia. However, usually it is found that the direct effects of nitrogen and phosphorus species on water quality are minimal compared with the indirect effects of these nutrients which result in the growth of excessive amounts of aquatic plants.
The excessive growth of these plants can significantly decrease water quality. For example, algae can cause such an increase in turbidity or reduction of light penetration in water that they have to be removed in water treatment. Generally their removal involves the addition of coagulants such as iron or aluminum salts followed by filtration through sand or other media filters. Whenever large concentrations of algae are present in the water, the rate at which these filters become clogged increases to such an extent that the filters have to be cleaned more frequently than they would if no algae were present.
The presence of algae and other plant remains in a water supply will increase the chlorine demand of the water since chlorine species will react with the remains of these organisms to form organochlorine compounds and oxidation products. It is believed by some that the presence of algae and other organisms in a water supply could protect pathogenic organisms from being destroyed by chlorine. For example, various kinds of higher animals could protect some pathogenic bacteria in their intestinal tract from destruction by chlorine normally added for disinfection purposes.
Probably the most significant effect of eutrophication of water supplies is the increased frequency of taste and odor problems. Many algae are notorious for causing tastes and odors in water supplies at very low concentrations of the organisms. A few cells per liter of some algae are said to impart a distinct odor to the water. Algal odors arise either from direct excretion of various organic compounds from the algae while they are alive or shortly after their death, or by the growth of various kinds of fungi on alga remains. The actinomycetes are such fungi; under certain circumstances, they seem to grow in large numbers shortly after the death of blooms of algae.
Some water-plant operators can tell the type of algae present in the water supply by their characteristic odors which may resemble the odors of cucumbers, pigpens, etc. The removal of these tastes and odors from water supplies often involves the addition of activated carbon and/or various kinds of oxidizing agents such as chlorine, ozone or permanganate in an attempt to either sorb or oxidize the odor.
In addition to causing odors and tastes, the growth of algae and aquatic plants can also increase the color content of the water. In general, dead algae release organic compounds which cause a pale yellow color in the water. The removal of color from the water often involves the addition of various oxidizing agents and coagulants with or without activated carbon for sorption of the organics.
Another effect of eutrophication on a water supply concerns eutrophic lakes where oxygen is depleted in the hypolimnion during summer stratification. The lack of oxygen in these deeper waters often causes large concentrations of iron, manganese and/or sulfide species to occur which in turn cause serious water quality problems. If this lake water is used for domestic or industrial purposes, the water-plant operator must take the warm epilimnetic waters which contain large concentrations of algae, instead of taking the cold hypolimnetic waters which are lower in algal numbers.
The location of the depth of a water-supply intake in a lake is dependent upon the degree of eutrophication of the lake. Generally, in oligotrophic lakes the over-all water quality is better in the hypolimnetic (bottom) waters than in the epilimnetic (surface) waters. The bottom waters are colder and contain less algae and particulate matter. However, in eutrophic lakes, the loss of oxygen from the bottom waters results in anaerobic conditions which lead to the formation of reduced species of iron, manganese and sulfur. The oxidized forma of iron (FeIII) and manganese (Mn IV) in natural water are the predominant forms in the presence of dissolved oxygen. Since these forms are insoluable as Fe(OH)3 and MnO2 in natural waters, they settle to the bottom of the lake and become incorporated in the sediments. Under oxidizing conditions, sulfur is present as sulfate and does not cause any water quality problems in most water supplies. In eutrophic lakes which experience the loss of all dissolved oxygen in the hypolimnion during periods of thermal stratification, anaerobic conditions develop that promote biochemical-chemical production of ferrous iron, manganous manganese and hydrogen sulfide. Since these reduced forms of iron and manganese are highly soluble, the concentrations may increase to levels which cause serious water quality problems.
From a water quality point of view for water supplies, the surface waters of all lakes tend to be of much poorer quality. However, in eutrophic lakes the presence of large amounts of iron, manganese or hydrogen sulfide causes serious water quality problems with the result that the water-supply intake must be adjusted to take the surface waters of the lake in order to avoid the high iron, manganese and hydrogen sulfide found in the bottom waters.
There are reports that organics such as those present in natural color cause an increased fouling of ion exchange resins. Although work in this area to date has not clearly demonstrated the relationship between frequency of fouling and eutrophication, it is reasonable to suppose that further studies may show that more eutrophic waters tend to foul ion exchange resins at a greater rate.
In summary, the eutrophication of domestic and industrial water supplies causes significant problems in water quality. In some cases, the seriousness of these problems can be measured by the increased treatment costs necessary to restore desirable water quality. For example, many water treatment plants keep accurate records of all chemical doses used. Eutrophication could conceivably be measured then by the amounts of chemicals that have been used at a given water plant in order to treat the water.
RECREATIONAL USES
Probably the most significant effect of eutrophication on recreational uses of water is with respect to its aesthetic quality. In general, excessive growths of aquatic plants cause a significant decrease in the aesthetic quality of the water. Certainly some plants, such as water lilies, are pleasing to the eye. However, when they become so thick in an area that it becomes impossible to swim, boat or fish, then their pleasing qualities rapidly become aesthetically undesirable.
These areas where the effects of growths of excessive amounts of aquatic plants are the most pronounced are usually the same areas where man's contact with the water is greatest. The nearshore environment-the shallow waters near the edge of a lake or stream-can become completely choked with various kinds of rooted aquatics and attached algae. The thickness of growth of these plants can make it almost impossible to use the water in this area for any purpose.
Under certain conditions, even the planktonic algal growth that develops in the middle of the lake can accumulate as thick mats of decaying algae along the shoreline. In severe cases, these mats can be so thick that turtles, ducks and other aquatic animals walk on top of them! Their high protein content results in hydrogen sulfide production which in turn develops into an extremely foul-smelling condition that is sometimes mistaken for the discharge of untreated municipal waste. The situation can become so severe on some lakes that shoreline property decreases in value, while the property away from the shore is increasing in value.
In addition to causing aesthetic deterioration of water used for recreational purposes, eutrophication can also cause a deterioration of a lake's fishery which, in terms of total production, depends on the total input of aquatic plant nutrients. Through the food web, the plant nutrients combine through photosynthesis to produce the aquatic plants which feed the aquatic animals which eventually become fish food. Most eutrophic lakes have the greatest production of fish. Eutrophication, in addition to increasing the total fish production in the lake, also changes the type of fisheries. This change is generally one of complete loss of the cold-water fishes such as trout and cisco and almost complete dominance of the less desirable warm water fish such as carp and bullheads. The loss of cold-water fisheries is the result of the depletion of oxygen below the thermocline during the summer stratification period. In an oligotrophic water, oxygen is lost during summer stratification below the thermocline so that a fish that would normally inhabit cold waters simply does not survive there. In summary, the effect of eutrophication on fisheries is one of increased total fish production which results in less desirable fish.
AGRICULTURAL USES
The eutrophication of a water can have significant effect on agricultural uses of the water. Some of the blue-green algae are reported to excrete highly toxic substances which can cause death of waterfowl, cattle, sheep and other animals. Although it is not known how frequently this occurs, some studies have shown that toxins produced by these algae could be significant in certain situations. With respect to human health, however it is estimated that the average human being would have to consume about 1 to 2 quarts of the "pea soup" that results from an excessive bloom of blue-green algae before he would be adversely affected.
Agricultural uses of eutrophic water are also affected where such water is transmitted in canals. The transmission of, water in irrigation canals can he greatly inhibited by excessive growths of aquatic weeds. The weeds increase the frictional resistance and, in addition, these same weeds can cause significant losses of water through increased evapotranspiration. This problem is particularly important in the arid areas of the western part of the United States where long irrigation water canals are used.
METHODS OF REDUCING THE EFFECTS AND RATE OF EUTROPHICATION
The eutrophication of a lake can be controlled or its effects on water minimized by reducing the nutrient input to the lake; increasing nutrient output from the lake, immobilizing nutrients within the lake and controlling excessive growths of algae and macrophytes within the lake. Various methods that have been proposed in each of these areas are summarized under the following topics.
NUTRIENT REMOVAL FROM WASTE WATERS
Since domestic waste waters represent a potentially significant source of aquatic plant nutrients, it is often the source that is looked to first for control. The nitrogen and phosphorus and, to some extent, the other trace elements can be removed from waste waters so that these waters will not be as fertile as they would have been if no nutrients were removed. Various laboratory pilot plant and field scale experiments have shown that both nitrogen and phosphorus can be removed from waste waters at a moderate cost. Phosphorus removal is accomplished by alum flocculation, ferric iron flocculation or lime precipitation, all three of which will remove 80 to 90 per cent of the phosphorus present in waste waters. Removal of ammonia and other nitrogen species can be done by such means as ion exchange, stripping ammonia at high pH in a gas stripping tower or bacterial denitrification in which ammonia is oxidized to nitrate which then becomes dentrified to form nitrogen gas. These various methods have been applied and can bring about nutrient reduction.
In general, however, these methods will cause a cost increase over conventional waste-treatment costs. At the present time, the cost of phosphorus removal is in the order of a few cents per thousand gallons depending on the size of the plant, with larger plants able to accomplish phosphate removal more inexpensively per unit size. This represents roughly a doubling of conventional waste-water-treatment costs. Nitrogen removal is now more expensive although it is expected that the cost will decrease if additional experience in treating water for nitrogen removal is obtained. It is likely that significantly large amounts of funds will soon be spent, first for phosphorus removal and then, in a few years, for nitrogen removal from waste waters.
DIVERSION OF WASTE WATERS
The most frequently used method to reduce lake eutrophication primarily caused by waste waters is to divert the waste waters around or away from the lake. This approach has been used in Madison, Wisconsin, and is being tried in Lake Washington in Seattle, Washington, and to some degree, in Lake Tahoe. While this method does eliminate the problem from the original lake that received the waste waters, it also increases the problem for receiving waters. From a long-range point of view, wastewater diversion is not a satisfactory solution to the problems of eutrophication. Wastewater treatment will have to be practiced as a means of controlling nutrients.
DREDGING
A procedure that can be used to remove nutrients from a lake involves dredging of lake sediments. As previously pointed out, a lake's annual process of self-fertilization and subsequent release of nutrients from sediments to overlying waters may, for some lakes, be one of the primary sources of the lake's nutrients. If nutrient-rich sediments could be dredged from the lake, it is possible that this self-fertilization would be decreased.
One of the problems with dredging, however, is that the nutrient content of lake sediments does not usually change drastically with depth. Recently conducted studies show that, for many lakes, phosphorus and nitrogen content of sediments is approximately the same at the surface as 10 to 15 feet below the surface, although there are some indications that the leachability of elements deep in the sediments may be somewhat less than the leachability of surface sediments. Therefore, it is reasonable to question whether or not dredging will have a significant effect on nutrient release. Certainly any undertaking in which dredging is proposed should be preceded by studies of nutrient release from the various types of sediments that will be dredged.
Another problem that usually accompanies the dredging of sediments is a shift from the growth of rooted aquatic plants to planktonic algae. This shift is due to the fact that rooted plants cannot develop in moderately deep water since algae in the overlying waters shut off the light available to them. As a result of dredging, a lake may be changed from one with an aquatic weed problem to one with an algal scum problem due to the growth of excessive numbers of planktonic algae.
Dredging of lakes clearly needs to be more properly evaluated before it can be used as an effective tool in eutrophication management. Care must be exercised in methods of disposal of the dredged material so that the nutrients present in these materials are not leached out and carried to a nearby watercourse. Considerable concern has been expressed about the effect of the disposal of material dredged from the Great Lakes and its harbors. The hopper dredges used to remove sediments from harbors do not remove large amounts of nutrients since the dredged materials are washed as part of the dredging operation to remove fine particles. Various studies have shown that these fine sediments contain the largest fraction of plant nutrients.
FLUSHING
Flushing can be effectively used to control nutrients within a lake. If large volumes of water low in nutrient content are available, flushing the lake with this water will increase water quality and decrease the frequency and seventy of algal blooms. However, lakes rarely have large quantities of nutrient-poor water available for flushing purposes.
ZONING
It is possible that one of the best methods to control nutrient flux for a given lake is to control land use within the watershed . For example marshes could be zoned to prevent their being dried up for agricultural or urban purposes. Zoning regulations could also control the type of farming and the types and amounts of fertilizers used in order to protect a given watershed.
CHEMICAL CONTROL OF NUISANCE PLANT GROWTHS
Both algicides and herbicides are being used to control the excessive growths of aquatic plants in natural waters. Algicides such as copper sulfate can effectively control algae. Herbicides such as 2, 4-D and sodium arsenite do control rooted aquatic plants. In general, the application of these chemicals to a water requires close attention and supervision, requires large expenditures of funds and must be done at frequent intervals during the growing season. On some occasions, it has been found that whenever a lake is treated to remove certain kinds of algae, a few weeks later another type of algae will rapidly develop and replace that which has been killed off. A potentially quite important aspect of the use of algicides and herbicides is the question of their effects on other organisms present in the water or on other uses of the water. If these chemicals persist in the water, there are always questions concerning their toxicity to fish, to fish-food organisms, or to man if the water is used for domestic or industrial purposes.
This toxicity question may be broken down into two parts. On the one hand, there is an acute toxicity that is associated with the application of the materials. If any of these chemicals are applied in excessive doses, they can cause fish kills. On the other hand, the more important question today, since acute toxicity can be readily taken care of by proper application, is one of chronic toxicity. Will the presence of these chemicals cause genetic changes? Are they carcinogenic; do they affect the rate of reproduction? These kinds of questions regarding long-lasting effects on the aquatic populations cannot be answered at the present time because of insufficient knowledge.
BIOLOGICAL CONTROL OF NUISANCE PLANT GROWTHS
The use of biological controls of excessive growths of algae and macrophytes has not been developed to the point where any potentially effective agents are likely to be found in the near future. The most promising results to date in this area have come from the work on the isolation of a virus that shows promise of being specific for blue-green algae. If such a virus can be found and readily cultured, it may be possible to use it to control excessive blooms of blue-green algae.
HARVESTING
Other methods of reducing the nutrient content or the flux to a given body of water include the removal of weeds, debris and rough fish and the harvesting of algae and aquatic weeds along the shore. Although harvesting does remove certain amounts of nitrogen and phosphorus, in general, it does not make significant inroads on the nutrient balance of a lake. Harvesting procedures are, however, justified in terms of improving the aesthetic quality of the lake by making it useful for swimming, boating and fishing. In situations where total nutrient input is low, harvesting may remove a significantly large proportion of nutrients from the lake and, therefore, would be justified as a nutrient removal procedure.
Many express the opinion that excessive growths of aquatic plants could lead to a harvestable crop of fish or other organisms. As previously explained, larger crops of fish are produced in eutrophic lakes; however, the large fish crops do not occur until sufficiently large populations of algae cause serious deterioration of water quality. In Florida, the manatee is used to keep some of the canals from becoming completely choked with macrophytes. While these animals clear the canals by consuming large quantities of aquatic plants, they are not able to remove significant amounts of nutrients from the water.
Although the harvested weeds do have a good fertilizer and humus potential and could be used as all agricultural fertilizer, the problems of handling and hauling them greatly restrict their use fur this purpose. Frequently the harvested aquatic weeds and rough fish must be hurled in landfills because no one wants the material.
MIXING OF LAKES
One of the methods that is sometimes proposed to control eutrophication is to destratify lakes with the use of compressed air or pumps. The basis of this method is that it is generally believed that aquatic plant nutrients such as phosphorus are not released under aerobic conditions. Recent studies at the University of Wisconsin Water Chemistry Laboratory have shown that this is not the case. Significant phosphorus release occurs in many types of lake sediments under aerobic conditions, and, therefore, the aeration of a body of water for the control of algae is of questionable value particularly if the aeration destroys the thermocline. The thermocline is an effective barrier to nutrient transport from the sediments in many lakes. Very small amounts of chemicals are transported across it. The thermocline is present throughout the growing season which is the critical period of the year for transport of nutrients from the sediments to the overlying water. Therefore, rather than mixing a lake to bring about complete oxygenation and to destroy the thermocline, it would be better to aerate the hypolimnion of a lake without destratification.
Special devices are available which enable this to be done. Hypolimnetic aeration with either air or gaseous oxygen allows the cold water fisheries to exist and would tend to control the nutrients to some degree at the same time. This method of aeration would reduce the amounts of nutrients circulated in overlying waters at fall overturn and would quite possibly take less energy than to completely mix a body of water.
CONTROL OF AGRICULTURAL SOURCES OF NUTRIENTS
Agricultural sources of nutrients can be controlled through educating the farmer in terms of when to apply nutrients, what concentrations to apply and how to control soil erosion and other conditions that tend to promote high nutrient fluxes from farmland. The manure problem that exists in Wisconsin and other nearby states can be controlled by having the farmer store the manure in tanks over the winter period and then spread it on the land after the ground has thawed in the spring.
URBAN DRAINAGE
Control of urban nutrient sources at the present time has little promise. The volume of storm waters that occur during normal thundershower activity is often such that it is impractical to consider diverting these waters or even treating them by any of the conventional means readily available today. The future in control here may lie in understanding the sources of nutrients and thereby controlling them at their source. One possible method is to decrease nutrient input to lakes by increasing street sweeping and effective leaf pick-up.
HYPOLIMNETIC WITHDRAWAL
Eutrophic lakes that are sufficiently deep to develop a stable thermocline accumulate higher concentrations of nitrogen and phosphorus in hypolimnetic waters than they do in the epilimnetic waters. The higher concentrations are derived from the sediments under anaerobic conditions. In some lakes, hypolimnetic withdrawal of water will result in a higher nutrient flux from the lake than would be obtained with epilimnetic removal of water.
In some cases where hypolimnetic withdrawal of water from eutrophic lakes has been practiced, serious hydrogen sulfide odor problems have developed near the point of water discharge due to the release of this gas from the anoxic waters of the hypolimnion of the lake.
WATER-LEVEL MANAGEMENT
Water-level manipulation in impoundments and, where possible, in lakes can reduce the crops of rooted aquatic plants. In cold climates a combination of lowering water levels and freezing will reduce macrophytes in lakes. However, lowering of water levels to achieve freezing or dessication may result in an increased release of nitrogen and phosphorus species.
IMMOBILIZATION OF NUTRIENTS
Aluminum and iron salts may be added directly to the lake to remove phosphorus from the lake water and carry it to the sediments. This method of nutrient control needs study to define the required chemical dosages and the necessary frequency of treatment.
RATE OF RECOVERY OF A LAKE UPON APPLICATION OF REMEDIAL ACTION TO CONTROL EXCESS NUTRIENT DISCHARGE TO A LAKE
The rate of recovery of an excessively fertilized lake after a reduction in nutrient input is dependent on many factors, the more important of which are the morphology of the lake including its drainage basin, the hydrology of the lake (relative amounts of surface and ground water), the theoretical flushing period of the lake, the amount of short-circuiting of its inlet and outlet waters, the amounts, forms and distributions of aquatic plant nutrients in the sediments of the lake, the magnitude of nutrient input reduction, the chemical composition of the water, the chemical processes occurring in the lake, etc. The interrelationship of the various factors is poorly understood. There is a tendency to use the hydraulic flushing characteristics of the lake as a basis for predicting the rate of recovery upon nutrient input reduction. For chemical species such as phosphorus, it is known that this approach grossly overestimates tile rate of recovery. The proper approach for estimating the rate recovery after phosphorus input reduction is to use a phosphorus residence lime model rather than the hydraulic residence time model Where such models have been applied, it is generally found that the phosphorus residence time model predicts a rate of recovery on the order of a year or two, while the hydraulic residence time model predicts a many-year rate.
CONCLUSIONS
Eutrophication of natural waters is one of the most significant water quality problems that man faces today. The growth of excessive amounts of aquatic plants can cause a significant decrease in water quality resulting in increased costs for water treatment, decreased quality for use in recreational purposes and a hindrance to the use of water for agricultural purposes. The nutrients that cause this excessive plant growth are nitrogen, phosphorus and other trace elements. These elements are derived from many sources, the most important of which are domestic waste waters, urban drainage, agricultural sources, the atmosphere, ground water and, to some degree, lake sediments.
The control of eutrophication can best be effected on point sources such as waste water effluent by nutrient removal. Other methods of control which show promise include removal of weeds, debris and rough fish, dredging of lake sediments, flushing and zoning. Chemicals such as herbicides and algicides can be used to provide temporary relief from excessive growths of aquatic plants. However, their use may result in significant water quality problems at some future date.
There is an urgent need for additional research in all aspects of eutrophication ranging from the hydrodynamics, hydrogeology, hydrobiology and water chemistry of lakes and streams to the various engineering aspects of nutrient control including the social, political and economic aspects of water resources management. The control of eutrophication of natural waters will require cooperative efforts of aquatic scientists, engineers, economists, social, political and legal experts. Only through the combined efforts of these individuals will it be possible to manage water resources for the betterment of mankind.
LITERATURE CITED
Anonymous. 1961. Algae and metropolitan wastes. Trans. Seminar on Algae and Metropolitan Wastes, Cincinnati, Ohio. R.A. Taft San. Engr. Center.
Anonymous. 1967. Environmental requirements of blue-green algae. Proc. Symposium, Univ. Washington and Fed. Water Pollution Control Admin. Pacific Northwest Water Lab.
Anonymous. 1967. Excessive water fertilization. Report to Water Subcomm. of Nat. Res. Comm. of State Agencies, Madison, Wis.
Anonymous. 1969. Effects of fertilizers on water quality. Nat. Fertilizer Develop. Center, Tenn. Valley Author.
Beeton, A.M. 1965. Eutrophication of the St. Lawrence Great Lakes. Limnol. Oceanog. 10:240-254.
Edmondson, W.T. 1968. Water-quality management and lake eutrophication: the Lake Washington case. In: Water Resources Management and Policy. Univ. Washington Press.
Foge, G.E. 1965. Algal Cultures and Phytoplankton Ecology. Univ. Wisconsin Press.
Frey, D.E. (ed.) 1963. Limnology in North America. Univ. Wiscorsin Press.
Fruh, E.G., K.M. Stewart, G.F. Lee and G.A. Rohlich. 1966. Measurements of eutrophication and trends. Jour. Water Poll. Control Fed. 38:1237-1258.
Goldman, C.R. (ed.) 1966. Primary Production in Aquatic Environments. Univ. California Press.
Hasler, A.D. 1947. Eutrophication of lakes by domestic drainage. Ecology 28:383-395.
Hutchinson, G.E. 1957. A Treatise on Limnology. Vol. I, Geography, Physics, and Chemistry. John Wiley and Sons, New York.
______________ 1967. A Treatise on Limnology. Vol.11, Introduction to Lake Biology and the Limnoplanklon. John Wiley and Sons, New York.
Jackson, D.F. (ed.) 1964. Algae and Man. Plenum Press.
_______________ 1968. Man and the Environment. Syracuse Univ. Press.
Lee, G.F. (chairman) 1966. Report on the nutrient sources of Lake Mendota. Lake Mendota Problems Comm., Madison, Wis. (revised 1969).
_______________ 1970. Factors affecting the exchange of materials between waters and sediments. Literature Review No. I, Eutroph. Inform. Program, Univ. Wisconsin.
Lee, G.F., and E.G. Fruh. 1966. The aging of lakes. Ind. Water Engr. 3:26-30.
Lewin, RA. 1962. Physiology and Biochemistry of Algae. Academic Press.
Machenthun, K.M. 1962. A review of algae, lake weeds and nutrients. Jour. Water Poll. Control Fed. 34:1077-loss.
1965. Nitrogen and phosphorus in water. An annotated selected bibliography of their biological effects. U.S. Dept. Health, Education and Welfare.
Machenthun, K.M., and W.M. Ingram. 1966. Algal growth factors other than nitrogen and phosphorus. Fed. Water Poll. Control Admin.
_______________ 1967. Biological associated problems in freshwater environments: their identification, investigation and control. Fed. Water Poll. Ccntrol Admin.
Machenthun, K.M., W.M. Ingram and R. Porges. 1964. Limnological aspects of recreational lakes. U.S. Dept. Ilcalth, Education and Welfare.
Middlebrooks, E.J., T.E. Maloney, C.F. Powers and L.M. Kaack. 1969. Proceedings of the eutrophication.biostimulation assessment work. Fed. Water Poll. Control Admin., Corvallis, Ore.
National Academy of Science. 1969. Eutrophication: Causes, Consequences and Correctives. Nat. Acad. Sci., Wash., D.C.
National Technical Advisory Committee (to the Secretary of the Interior). 1968. Water quality criteria. Fed. Water Poll. Control Admin.
Sawyer, C.N. 1966. Basic concepts of eutrophication. Jour. Water Poll. Control Fed. 38:737-744.
Sawyer, C.N., J.B. Lackery and A.T. Lenz. 1945. Investigation of the odor nuisances in the Madison Lakes, particularly Lakes Monona, Waubesa and Kegonsa, from July 1943 to July 1944. Report to Governor's Committee, Madison, Wis.
Stewart, K.M., and G.A. Roblich. 1967. Eutrophication - a review. Calif. State Water Quality Control Bd., Pub. 34.
Strickland, J.D.H. 1960. Measuring the production of marine phytoplankton. Fish. Res. Bd. Canada, Bull. 122.
Veatch, J.O., and C.R. Humphrys. 1964. Lake terminology. Dept. Res. Develop., Agric. Exp. Sta., Mich. State Univ.
Vollenweider, R.A. 1968. Scientific fundamentals of the eutrophication of lakes and flowing waters, with particular reference to nitrogen and phosphorus as factors in eutrophication. Organ. Econ. Coop. and Develop., Directorate for Scientific Affairs.
_____________ 1969. A manual on methods for measuring primary production in aquatic environments. International Biological Programme, Handbook 1 2.
Weibel, S.R., R.J. Anderson and R.L. Woodward. 1964. Urban land runoff as a factor in stream pollution. Jour. Water Poll. Control Fed. 36:914-924.
NOTE
Originally prepared for the Supplement to the Encyclopedia of Chemical Technology, John Wiley and Sons (1970), and printed here with their permission. Also published in essentially this form as Occasional Paper No. 2 of the University of Wisconsin Eutrophication Information Program.
References as:"Lee, G.F., 'Eutrophication,' Transactions of the Northeast Fish and Wildlife Conference, pp 39-60 (1973). "

|
|