The increasingly stringent requirements being placed on domestic water supply finished water quality are causing water utilities and regulatory agencies to give greater consideration to the possibility of managing water supply contaminants at the source. This paper reviews several aspects of the information available on the potential for controlling domestic water supply water quality by source pollutant control. Consideration is given to both surface and groundwater systems. Emphasis is given to the control of raw water quality problems due to excessive growths of algae, trihalomethane precursor sources, and the protection of groundwater quality from landfill leachate. Particular attention is given to the Sacramento-San Joaquin River Delta system which serves as a domestic water supply for approximately 20 million people in California.
Increasing concern is being focused on the use of copper sulfate as an algicide in water supply reservoirs because of the finding that some cities have "excessive" amounts of copper in their wastewater discharges from this source. The "excessive" concentration is based on a comparison with US EPA water quality criteria. It is well known that copper exists in many forms, only some of which are toxic. It has been found that in a number of situations the copper in surface waters is in a nontoxic form. The US EPA criteria however consider all forms of copper to be equally toxic. It is therefore important for water utilities that utilize copper for algae control to work with state regulatory agencies to be certain that the copper water quality standards adopted for the municipalities' wastewater discharges properly focus on the control of copper that leads to toxic forms in the receiving waters.
It has been found that several water utilities and agencies that use Delta waters as a raw water source are experiencing significant algal related water quality problems, including tastes and odors, and increases in trihalomethane precursors. The preliminary calculations show that it may be possible to significantly reduce the growth of algae in the Delta and in down-Delta water supply reservoirs as well as the aqueduct system transporting waters from the Delta to the southern part of the state through limiting phosphorus input to the Delta by treating domestic wastewaters for phosphorus control.
A discussion is presented on the impacts of eutrophication of Lake Tahoe on the use of this waterbody as a source of domestic water supply and on the approach that should be considered to manage the excessive algal growths that are occurring within this waterbody that lead to water supply taste and odor problems. The growth of algae in Lake Tahoe is limited by the nitrogen loads to the lake. These loads have been increasing over the years. Nitrogen is primarily derived from atmospheric sources through precipitation to the lake's surface. The primary source of atmospheric nitrogen in the Lake Tahoe basin is automobile, bus, and truck engine exhaust discharge of NOx. It is also concluded that the fertilization of lawns and other shrubbery, including golf courses, within the Lake Tahoe basin is leading to significant growths of attached algae in the nearshore waters of the lake. The fertilizers are transported via groundwater to the nearshore waters of the lake. It appears that these growths may be contributing to the domestic water supply water quality problems that water utilities using Lake Tahoe water as a source have been experiencing in the past few years. In order to protect domestic water supply water quality it is recommended that water utilities that utilize Lake Tahoe as a raw water source work aggressively toward limiting automobile and other internal combustion engine vehicular traffic in the Lake Tahoe basin. Further, water utilities should also aggressively pursue banning all lawns and lawn and shrubbery fertilization within the Lake Tahoe basin.
It has been found that the current implementation of regulations governing land disposal of wastes in municipal landfills is not adequate to protect groundwater quality in California. The current approach of using plastic and compacted clay liners for landfills postpones the water pollution problems; it will not prevent them. A guide is provided to municipal water utilities and agencies on the approach that they should adopt to provide for far greater groundwater quality protection from landfill leachate and other sources of pollutants than is being achieved today.
Introduction
Municipal water utilities are facing ever increasing demands to improve finished water quality. This situation is causing many water utilities to initiate new treatment processes or approaches as well as to improve the performance of existing treatment works. These various improvements are adding to the cost of producing a potable and palatable domestic water supply. Many water utilities are finding that increased urbanization and industrialization of their water supply watersheds is causing increased contaminant loads that must be removed in the treatment works. Both of the above mentioned factors are causing water utilities and water quality regulatory agencies to consider the feasibility of controlling domestic water supply water quality by controlling contaminant concentrations at the water supply source. This paper presents an overview of the current information on some programs that have been, or could potentially be, successful in improving domestic water supply raw water quality.
Eutrophication of Domestic Water Supply Lakes and Reservoirs
The eutrophication (excessive fertilization) of domestic water supply lakes and reservoirs is a well known cause of water supply water quality deterioration. The growth of planktonic algae in domestic water supplies is known to cause increased tastes and odors, shortened filter runs, increased chlorine demand, increased turbidity, and, for some situations, increased trihalomethane (THM) precursors. Gilbert (1991) reported that surveys taken of consumer satisfaction with a domestic water supply aesthetic quality found that for the East Bay Municipal Water District, about 70 percent of the respondents indicated that they found that their water supply aesthetic quality was satisfactory. For the San Francisco Bay region as a whole, consumer satisfaction was about 35 percent. For the state as a whole, it was about 25 percent. Since taste and odor problems are one of the primary causes of consumer dissatisfaction with water supply water quality and since in California water supply taste and odor problems tend to be of algal origin, it is clear that algal growth in surface water supplies in this as well as other states is a frequent cause of significant algal-related taste and odor problems. For additional information on the impact of algae on domestic water supply taste and odors and other water quality problems, consult Palmer (1959).
Controlling Algal Growth through the Use of Copper Sulfate
Many water utilities that depend on impounded surface water as a supply have for many years been practicing algae control through the use of toxic chemicals, such as copper sulfate, to kill algae. It is generally possible for water utilities through an aggressive raw water supply water quality monitoring program to detect the early stages of an algal bloom (large number) before the algae develop in sufficient numbers to cause serious raw water quality deterioration. At that time, it is possible to use copper sulfate to control the algal bloom before it develops to the degree that causes severe domestic water supply water quality problems. Jones and Lee (1982) provided guidance to water utilities on the type of raw water quality monitoring program involving the measurement of algal chlorophyll that water utilities should practice in order to minimize water quality problems caused by excessive growths of algae.
With the efforts of the US EPA and many state water pollution control agencies to control toxic chemicals in wastewater effluents, the use of copper sulfate as an algicide in domestic water supply reservoirs is being increasingly questioned as a result of some municipalities, such as New York City, finding that the primary source of copper in the City's wastewaters and New York Harbor is its use for algae control in its water supply reservoirs. A similar situation appears to be occurring in South San Francisco Bay where "excessive" concentrations of copper are being found in Bay waters compared to US EPA water quality criteria and state of California proposed water quality objectives. It is alleged that this copper is derived at least in part from the use of copper as an algicide in water supply reservoirs for communities that discharge their wastewaters to the Bay. It is now becoming apparent that the continued use of copper sulfate for algae control will have to be much more judiciously practiced than has occurred in the past where the residual copper cannot be carried to any significant extent into the distribution system and thereby become part of the city's wastewater discharges.
An important aspect of this situation that should be considered is that in both New York Harbor and South San Francisco Bay, the "excessive" copper compared to aquatic life water quality criteria and standards is non-toxic to sensitive forms of aquatic life. Such a situation can readily lead to water utilities having to reduce the use of copper for algae control in the name of protecting aquatic life in receiving waters for the wastewater discharges, yet have little or no impact on aquatic life in the receiving waters for the copper derived from municipal water utility use. This is a result of the fact that copper exists in a variety of chemical forms, only some of which are toxic to aquatic life. Certain waterbodies, such as shallow marine bays, tend to convert copper to nontoxic forms.
The above described situation as well as other similar situations, where chemicals used in water treatment practices are becoming pollutants in receiving waters, will require that water utilities take a much more aggressive approach toward helping to develop technically valid, cost-effective water quality criteria and standards-objectives. The State Water Resources Control Board staff has proposed the use of US EPA water quality criteria as a basis for state water quality objectives. As discussed by Lee and Jones (1990), such criteria are based on worst case or nearly worst case assumptions and therefore in most situations are overly protective of aquatic life compared to what could be achieved if more appropriate criteria and objectives were utilized.
Because of the concern about the toxicity of copper to aquatic life in lakes and reservoirs used for recreational purposes and/or the cost of treating some lakes and reservoirs with copper sulfate, many water utilities could not or do not practice algal control through controlling algal blooms with copper sulfate. While typical eutrophication control programs based on reduction of algal nutrient input to a lake or reservoir that have been adopted across the US focused primarily on managing the impacts of algae on recreational use of the waters where the algal related problems were floating scum, decaying algae on the beach, malodorous conditions, low light penetration, dissolved oxygen depletion in hypolimnetic (bottom) waters, fish kills, etc., one of the benefits of such programs has been improvement in the domestic water supply raw water quality. With increasing constraints on water utilities' use of copper sulfate, water utilities should give greater consideration to controlling algal growth in their lake or reservoir water supply by limiting algal nutrients added to the waterbody from its watershed.
In the early 1980's, Lee and Jones, working through the American Water Works Association Quality Control in Reservoirs Committee, attempted to have the manager of the US EPA's "Clean Lakes Program" include within the scope of this program the protection and improvement of domestic water supply raw water quality. The manager of the program at that time in Washington, DC indicated that this was inappropriate. Subsequently, however, under new management, the program apparently considers the benefits of improving domestic water supply raw water quality as part of the justification for lake remediation programs supported by the agency. This situation may provide the opportunity for water utilities to gain some funding from federal and state sources for nutrient control programs.
Lee and Jones (1988a) presented a comprehensive review on the North American experience in eutrophication control through phosphorus management. As they discussed, with few exceptions, it has generally been found throughout the world that controlling the phosphorus input to a freshwater lake or reservoir can, if practiced to a sufficient extent, reduce the amount of algae that would develop in the waterbody. Since typically algal related domestic water supply water quality problems are related to the numbers of algae present, reducing algal biomass in a water supply reservoir is in the direction of reducing domestic water supply raw water quality problems due to algae. There are, however, significant differences in the ability of various types of algae to cause domestic water supply water quality problems. Certain types of algae are well known for their highly obnoxious, very potent odors associated with their presence in a water; this is especially true for certain blue-green algae which are known to have odors that are characterized as "pig-pen" like. Normally, however, it is found that reducing the overall nutrient (phosphorus) loads to a lake or reservoir tends to be in the direction of not only reducing total algal biomass, but also reducing the frequency and severity of highly obnoxious algal blooms. For further discussion of this topic, consult Lee (1973).
Lake Tahoe Water Quality
The use of Lake Tahoe as a domestic water supply source provides an unusual example of the potential involvement of water utilities in managing eutrophication of a lake or reservoir through limiting nutrient inputs to the waterbody. Recently the authors have completed a review of the available information on the factors controlling algal related water quality in Lake Tahoe (Jones and Lee, 1990). The majority of this data was developed by Dr. Goldman and his associates at the University of California at Davis and the Lake Tahoe Research Group. It was found that both the phytoplankton (open water suspended algae) and the periphyton (nearshore attached algae) have been increasing in numbers with a concomitant adverse impact on the lake's water quality. Based on decreased Secchi depth (water clarity) and primary productivity, the numbers of planktonic algae have been increasing significantly in the open waters of the lake. (See Figures 1 and 2.) Similarly, although not as well documented, increased growth of periphyton is occurring in nearshore waters.
Lee, et al., (1978) and Rast and Lee (1978) developed a relationship between planktonic algal chlorophyll in lakes and reservoirs and Secchi depth where increased algae causes reduced light penetration. It is clear from the data of Goldman and others that while Lake Tahoe is ultra-oligotrophic and is one of the clearest lakes in the world, increased algal growth is occurring in this lake that is significantly reducing light penetration in the water column.
During the past several years some water utilities using Lake Tahoe as a raw water source have been experiencing significant problems with algal related tastes and odors. At this time it is not clear whether the problem is due to planktonic algae or attached algae that have broken off from their attachment or a combination of both. Some water utility personnel feel that this problem may have been exacerbated by the low water levels that have occurred in Lake Tahoe over the last few years. Additional work will have to be done to determine the relative role of planktonic algae, attached algae, and low water levels to sort out the specific causes of the taste and odor problems.
In the review by Jones and Lee (1990) it was found that the growth of planktonic algae in the lake is primarily controlled by the input of nitrogen to the lake. Using the techniques described by Jones and Lee (1986) and Rast and Lee (1984) to determine sources of nutrients for the lake, Jones and Lee (1990) concluded that the primary source of nitrogen which is stimulating algal growth is from the atmosphere and that based on the NOx emissions from vehicular exhausts in the Lake Tahoe Basin, it is concluded that automobile, bus, and truck traffic within the Lake Tahoe watershed is the primary source of nitrogen that is causing the increased algal growth in the lake.
Table 1 presents estimated nitrogen loads for Lake Tahoe for about 1950 (predevelopment) conditions and today. The predevelopment nitrogen loads to Lake Tahoe are estimated to be about 7 metric tons per year while today the total nitrogen load is about 100 metric tons per year. The most significant increase has been in the atmospheric nitrogen sources with direct precipitation on the lake's surfaces being the primary source of nitrogen for the lake. According to the Air Resources Control Board (1987) data (Table 2), vehicular traffic contributes about 2,500 metric tons per year of NOx to the atmosphere. This is equivalent to about 700 metric tons of nitrogen per year. It is therefore evident that automobile, truck, and bus exhaust discharges of NOx are highly significant sources of nitrogen for Lake Tahoe.

Jones and Lee also concluded that the Lake Tahoe Regional Planning Agency's (TRPA) Individual Parcel Evaluation System (IPES), which is being used to control population growth in the basin, is technically invalid and is not protecting the lake's water quality. The IPES score is agrowth limiting mechanism used by TRPA for the purpose of protecting lake water quality. The IPES score on a property is not related to the amount of nitrogen or, for that matter, other forms of algal available nutrients that ultimately reach the lake from that property. Jones and Lee recommended that in order to begin to effectively slow down the rate of deterioration of the lake water quality that is related to algal growth in the open and nearshore waters of the lake, aggressive action should be immediately taken toward greatly reducing, if not essentially eliminating, the use of internal combustion engine based automobiles, trucks, and buses within the Lake Tahoe watershed.
Jones and Lee also concluded, based on the work of others and personal observations, that part of the periphyton growing in the lake is due to nutrients derived from fertilizers used on lawns and shrubbery, including golf courses, etc. A significant part of the fertilizers used for landscaping purposes by public and private interests is being carried by groundwater to the nearshore waters of the lake where it stimulates periphyton growth in the region where the groundwaters enter the lake as submerged springs. Jones and Lee recommended that all lawns, including golf courses, and fertilized shrubbery be banned in the Lake Tahoe watershed. The basin should be allowed to return to native vegetation that does not require fertilization and/or irrigation.
While at this time domestic wastewater disposal is not allowed within the Lake Tahoe watershed, i.e., the system is sewered with the wastewaters exported out of the watershed, it is highly likely that previous wastewater disposal practices could be significant sources of nutrients for some nearshore areas of Lake Tahoe contributing to localized algal related problems in these areas. Nutrients derived from the previous use of septic tank wastewater disposal systems and wastewater spray irrigation disposal systems are, or could be, significant sources of nutrients which stimulate algal growth in some parts of the nearshore waters of Lake Tahoe. Jones and Lee suggested that additional work needs to be done to determine the potential significance of past wastewater disposal practices within the Lake Tahoe Basin as a source of nutrients for nearshore water quality problems. If there is interest in controlling excessive periphyton growth in a particular part of the nearshore area of the lake where the nutrients contributing to the excessive growth in that region are significantly derived from past wastewater disposal practices, it may become necessary to intercept the groundwater before it reaches the lake by pumping and treating the groundwater to remove the nutrients.

Table 1: Lake Tahoe Estimated N Load (tonnes N/yr) [After Jones and Lee, 1990]
|
Source |
Pre-Development |
Now |
|
Atmosphere - onto Lake Surface |
2.5 |
~100 |
|
Surface Water Runoff |
4 |
16 |
|
Groundwater |
0.5 |
2 |
|
Total N Loads |
7 |
118 |
Table 2: Estimated Contributions of NOX from Motor Vehicles [After Jones and Lee, 1990; Source: Air Resources Control Board, 1987]
|
tonne NOX/yr |
|
|
Automobiles |
800 |
|
Light & Medium Trucks |
630 |
|
Heavy Duty Trucks |
1160 |
|
Total |
2500 |
If the algal tastes and odors continue to persist, the water utilities using Lake Tahoe as a source should become proponents of significantly curtailing internal combustion engine based vehicular traffic within the Lake Tahoe Basin and eliminating the use of lawn and shrubbery fertilizers and irrigation within the basin as part of a domestic water supply source water quality control program. There can be little doubt that, if aggressive action is not taken in the near future in these areas, the frequency and severity of algal caused tastes and odors and other domestic water supply water quality problems will increase.
Impact of Water Supply Intake Location on Water Quality
Lee and Harlin (1965) discussed the benefits that water utilities could potentially develop in improved raw water quality by having lake or reservoir intake works designed so that water can be taken from various specified depths at certain times during the year. For more eutrophic waterbodies, it is often found that algal blooms tend to occur near the surface, where the numbers of algae and their potential impact on raw water quality decrease significantly with depth. This is especially true during the summer months when the waterbody may be thermally stratified. It therefore is possible that a water utility that has the option of taking water at various depths in a lake or reservoir (see Figure 3) could significantly improve the raw water quality that is influenced by algae by selecting water intake depth to minimize algal-related problems, such as tastes and odors, shortened filter runs, THM precursors, and anoxic waters with the associated elevated concentrations of iron, manganese, and sulfide. For such a program to be successful, however, the water utility will need to conduct a fairly intensive reservoir monitoring program to gain an understanding of how raw water quality changes with depth at various times of the year and under various meteorological conditions. Further information is provided on this topic by Lee and Harlin (1965).
Eutrophication of Rivers Used as Domestic Water Supplies
While the eutrophication of lakes and reservoirs' impact on domestic water supply water quality and its potential control by limiting phosphorus input to the waterbody are well known, the eutrophication of rivers and its effects on domestic water supply water quality are not well understood. There is no question, however, about the fact that algal growth in rivers can cause severe water quality problems for domestic water supplies. Rivers frequently carry relatively large numbers of algae. The difference between rivers and lakes and reservoirs however is that the algae present in a lake or reservoir are more readily discernible to the public because of the more quiescent conditions that typically exist in lakes and reservoirs compared to the turbulent conditions that frequently occur in rivers. Further, many rivers tend to be highly turbid due to inorganic turbidity derived from the transport of erosional materials. Such turbidity masks the presence of algae and may under severe conditions limit their growth due to reduced light penetration.
In some unpublished work by the senior author conducted on the upper Ohio River in 1960-61, it was found that the passage of elevated concentrations of algae in the river by a water supply intake caused the water utility to experience increased tastes and odors, shortened filter runs, etc. Slugs of algae that were present in the Ohio River arose from growth in the river as well as growth in reservoirs that served as the source of water for the river. If water utilities would monitor the planktonic algal chlorophyll in their river water supply and correlate this with algal related water quality problems, in many instances, they would find sufficient correlation to cause considerable justification for controlling nutrient inputs to rivers from upstream sources. There also may be sufficient justification in some situations to cause the managers of head water reservoir systems to be cognizant of the fact that, if they release surface water from a reservoir that has a high concentration of algae, this water may cause significant water quality problems for down river utilities. It should be possible in many multiple reservoir management situationsto include consideration of domestic water supply water quality as it relates to algal growth in the reservoirs and the release of reservoir water to the river in developing reservoir release programs.

THM Precursor Sources and Their Control
One of the most significant water quality problems for domestic water supply utilities that utilize surface waters as a source is the formation of THM's in the waters disinfected by chlorine or other strong oxidants, such as ozone in the presence of bromide. THM's arise from chlorine (primarily free chlorine) reacting with dissolved and particulate organic matter present in the raw water to form a group of low molecular weight halogenated hydrocarbons, such as chloroform. In the presence of bromide in the raw water, strong oxidants, such as free chlorine and ozone, oxidize the bromide to bromine. The bromine in turn reacts in a similar manner to free chlorine, forming brominated THM's.
The presence of bromide in a water supply is of particular significance as a THM precursor because it is much heavier than chlorine and therefore, since the THM MCL (maximum contaminant level) is based on a mass per volume concentration, a brominated THM is a much more important species than its equivalent chlorinated form with respect to meeting the MCL. It also appears that bromine may be a more effective halogenating agent than chlorine with the result that higher THM levels on a molar basis are formed when bromide is present compared to when it is absent. Bromides are frequently associated with seawater and brines. It is therefore obvious that water utilities with any sources of controllable bromide within their raw water supply should aggressively require control of those sources to the maximum extent possible.
Until recently, few water utilities determined the bromide concentration of the raw water supply with the result that there is very limited information available today on the pollution of water supplies by bromide. For seawater systems, the chloride to bromide ratio in accord with the law of constant relative proportions is a fairly well-defined ratio of about 0.003. As a result, for freshwaters contaminated with seawater, such as occurs in part of the San Joaquin-Sacramento River Delta (Delta), it is possible to estimate the bromide concentration of the water based on the chloride concentration. For other sources of bromide, however, such as an oil field or other brines, the seawater ratio may not be applicable to waters contaminated by brines from other sources. Caution should therefore be exercised in a complex system such as the Sacramento-San Joaquin River Delta system in assuming that all tributaries of the Delta have chloride to bromide ratios the same as seawater. While this could be the case, since the export of Delta water contaminated with seawater results in some of this water being returned to the Delta through the San Joaquin River system, it is important to verify, for this and other similar situations, that chloride concentrations can be used to estimate the bromide content of the water.
There is considerable justification for limiting the amount of seawater that enters the Delta in order to reduce the bromide input to this system and to reduce the potential for brominated THM formation. In the fall of 1990, the State Water Resources Control Board Delta Municipal and Industrial Water Quality Work Group made a recommendation to the California Water Resources Control Board to manage water quality within the Delta system so that the freshwater outflows from the Delta to the San Francisco Bay system will be sufficient to limit the saltwater migration into the Delta for the purpose of controlling the introduction of bromide in the seawater into Delta waters that are exported or used directly for municipal water supply sources. This is a highly justified source water quality control effort that is under review by the State Water Resources Control Board at this time.
Another example of a situation where bromide control in a source water was highly justified occurred in the work that the authors did with the Canadian River Municipal Water Authority, which utilized Lake Meredith in West Texas as a domestic water supply source (Lee and Jones, 1983). This lake received brine drainage to tributaries. This brine was derived from natural sources in the Canadian River watershed. It contained elevated concentrations of bromide which led to elevated brominated THM's in water supplies that use Lake Meredith water as a source. Efforts were made by the Canadian River Municipal Water Authority to control the amount of brine input to the tributaries of the reservoir for the purpose of limiting brine, and specifically bromide, input to the waterbody.
While it has been known for many years that controlling the concentration of organic precursors of THM's by their removal in treatment works can control THM concentrations, surprisingly little attention has been given to attempting to understand, and where possible control, organic THM precursors at the source. This is an area that deserves attention and that could be a potentially significant approach that could be utilized by some water utilities for controlling excessive THM's. The work of Randtke and his associates (Randtke, et al., 1988) has provided some insight into the potential sources of THM precursors. Table 3 presents a summary of Randtke, et al. data on the concentrations of THM precursors as measured in a standardized chlorination test (THMFP-trihalomethane formation potential) in various runoff waters and samples of effluents, etc. It is readily apparent from this and other work that certain types of land use and wastewater discharges are particularly significant sources of THM precursors. Randtke, et al. (1988) found that while THM precursor concentration in waters from various sources varied greatly, the THM yield as measured as THMFP concentration per mg carbon was remarkably constant. This points to the potential that for many situations controlling the total organic carbon (TOC) content of the water is a potentially reliable basis for controlling THM precursors. Obviously, there is need for additional study of the applicability of the Randtke, et al. results to other areas to be certain that the relatively constant ratio that they found between THM formation potential and TOC is found in other areas. There is some indication in the literature that this may not be the case. Water utilities and water pollution control agencies would therefore need to make an evaluation of this relationship of potentially significant sources of organic THM precursors in their watersheds in order to determine if the THM precursor source control program could be focused on controlling TOC discharge to waters that are tributary to the water supply source for the water utility.
Table 3: THM Formation Potential in Runoff and Point-Source Samples
|
Site Description |
DOC (mg/L) |
TOC (mg/L) |
THMFP (ug/L) |
|
Urban-Commercial |
17.8 |
67.0 |
1,152 |
|
Industrial Landfill |
257.0 |
337.0 |
1,555 |
|
Construction Landfill |
6.06 |
19.9 |
967 |
|
Terraced/Tiled Farmland |
4.52 |
203.4 |
4,329 |
|
Non-terraced Farmland |
4.92 |
7.55 |
400 |
|
Burned Bromegrass Land |
6.39 |
7.93 |
409 |
|
No-till Farmland |
4.72 |
7.09 |
482 |
|
Cattle Feedlot |
64.1 |
382.7 |
13,482 |
|
Tilled Farmland |
16.7 |
29.6 |
1,651 |
|
Swine Feedlot |
13.0 |
26.5 |
1,383 |
|
Cattle Feedlot |
71.1 |
122.5 |
4,747 |
|
Soybean Field |
9.92 |
13.6 |
710 |
|
Corn Field |
3.74 |
17.2 |
591 |
|
Corn Field |
13.4 |
17.3 |
1,008 |
|
Urban Construction |
3.03 |
22.7 |
1,486 |
|
Urban Residential |
5.12 |
6.99 |
395 |
|
Industrial Park |
4.48 |
6.72 |
274 |
|
Shopping Center |
3.09 |
5.22 |
268 |
|
Municipal Secondary Effluent (Activated Sludge) |
9.15 |
9.57 |
304 |
|
Municipal Secondary Effluent (Stabilization Pond) |
16.4 |
41.4 |
1,093 |
|
Municipal Secondary Effluent (Stabilization Pond) |
32.7 |
55.3 |
926 |
|
Municipal Secondary Effluent (Stabilization Pond) |
28.4 |
54.2 |
1,027 |
|
Sanitary Landfill Runoff Pond |
3.0 |
3.6 |
154 |
|
Refinery Effluent |
26.9 |
42.0 |
2,071 |
|
Cellophane Manufacturing Effluent |
5.4 |
8.3 |
272 |
|
Power Plant Cooling Water |
7.0 |
8.4 |
315 |
|
Power Plant Cooling Water |
7.0 |
7.8 |
340 |
|
Power Plant Ash Pond Influent |
3.7 |
3.9 |
151 |
|
Power Plant Ash Pond Effluent |
4.7 |
6.1 |
421 |
|
Electroplating Plant Effluent |
9.4 |
10.7 |
156 |
|
Meat Packing House Effluent |
16.1 |
20.8 |
819 |
|
Fertilizer Plant Wastewater Pond |
11.2 |
16.2 |
242 |
THM Yields of Runoff Samples
|
Sample Group |
Number of Samples |
Average THMFP (umoles/mgC) |
|
All Samples |
18 |
0.37 +/- 0.14 |
|
Urban Runoff |
6 |
0.39 +/- 0.14 |
|
Agricultural Runoff |
11 |
0.39 +/- 0.11 |
|
Feedlot Runoff |
3 |
0.35 +/- 0.07 |
|
Farmland Runoff |
8 |
0.41 +/- 0.12 |
After Randtke et al. (1987)
While THM organic precursors are derived from natural sources, such as decaying vegetation, etc., the activities of man through municipal and industrial wastewater discharges and agricultural run-off and drainage can significantly increase the THM precursor concentrations in a water supply. If a much better understanding existed of THM precursor sources and the amounts of precursors derived from various types of land use, then it might be possible to develop approaches that could effectively reduce precursor input. An example of this type of work is currently underway in the Delta by the California Department of Water Resources (DWR) with a paper on the results of this work was presented by Woodard (1991). The DWR study focuses on Delta sources of THM precursors. It, however, does not go far enough back into the tributary sources of the Delta to understand the specific sources of THM precursors that exist in the major tributaries to the Delta upstream of the Delta. It is clear from the Department of Water Resources monitoring data (DWR, 1989) that a significant amount of THM organic precursors are brought into the Delta from tributary sources to the Delta. DWR found that the five-year (1983-87) median (THMFP's) at Greene's Landing on the Sacramento River was 260 ug/L. At Vernalis on the San Joaquin River it was 450 ug/L, while the five-year median at the bank's export point was 490 ug/L. While it would be necessary to actually compute input loads of THM organic precursors from the Sacramento and San Joaquin rivers based on concentrations and flow data, it is clear that a significant amount of THMFP's are added to the Delta each year from Delta tributary sources and that a significant effort should be made to understand the specific contributions of these various sources since it could lead to the development of control programs that could influence THM formation in water supplies that use the Delta as a water supply source. It is therefore evident that the DWR current studies in this area should be expanded to include not only the definition of in-Delta sources but also upstream of the Delta sources of THM organic precursors.
It has been known for some time from work in various parts of the US that waters in contact with high organic soils, such as peat, which occur in some parts of the Delta, can have greatly elevated concentrations of organic THM precursors. From a review of the Department of Water Resources' monitoring data on waters added to and taken off of agricultural lands within the Delta, it has been found by the authors that the waters diverted from the Delta channels to agricultural lands and then pumped back to the channels will typically show a 1000 to 1500 ug/L increase in THM formation potential. It is evident from examination of the total dissolved solids (TDS) in the waters diverted from the channels to agricultural lands and the waters pumped back to the channels from these lands, that there is about a 2 to 3-fold evaporative concentration of salts on some of the agricultural lands within the Delta. This could mean that on the order of half of the increase in THM precursors discharged from Delta agricultural lands to the channels could be derived from evaporative concentration on the agricultural lands. The other half would be derived from leaching from peat soils and any crop or other plant residues present in or on the soil. It is likely that there is some change in the type of compounds that make up the organic precursors derived from the agricultural lands due to sorption, microbial transformation, and desorption-solubilization processes; and therefore, the chemical makeup of the dissolved organic carbon (DOC) added to agricultural lands will likely be different from that discharged from them. This could affect the relationship between DOC and THM formation potential since only a small part of the DOC is converted to THM's during disinfection processes involving chlorine or other strong oxidants in the presence of bromide.
It is recommended that an aggressive program be developed to reduce the amount of organic THM precursors added to Delta waters from agricultural as well as other sources. The first step in developing such a program is to better understand the relative significance of each potentially significant source for the Delta in each of its major tributaries. This program should include determination of specific sources of THM precursors that contribute more than about 10% of the total to a Delta tributary as well as within the Delta. These sources should in turn be investigated to understand what are the specific sources of THM precursors within the source and what potential control programs could be developed to reduce the amount of THM precursors present in the raw water supplies for the utilities that utilize water from the Delta. Similar kinds of programs should be conducted by water utilities throughout the country who face problems with excessive THM formation.
Ultimately, it should be possible to develop THM precursor export coefficients similar to the export coefficients that have been developed by Rast and Lee (1983) for nitrogen and phosphorus where certain types of land use or drainage would be expected to contribute certain amounts of THM precursors on a unit area per unit time basis. This would require determining the concentrations of THM precursors from various types of sources at fairly frequent intervals of one to no more than two weeks over at least a one- and preferably two-year time period while the flow of the source is also being measured. The objective of such measurements would be to develop mass THMFP per hectare per year data for runoff samples. For effluent samples, the total mass loading of THMFP's per year would be determined. This could in turn be potentially related to a population equivalent for municipal wastewaters which reflects the type and degree of treatment provided by the treatment works. For industrial wastewaters, it should be possible to develop a THMFP equivalent per unit of manufactured product or some other similar basis which relates the wastewater loads to industrial activity. It should be readily possible to determine a relationship between TOC removal in a wastewater treatment plant for certain types of wastes and a THMFP removal ratio.
The development of THM precursor export coefficients could be highly instrumental in having regulatory agencies to start to control municipal, industrial, and agricultural activities that represent significant sources of THM organic precursors for domestic water supplies. THM precursors in wastewaters, urban and agricultural drainage, etc. will ultimately be considered pollutants that have to be controlled through discharge permits in much the same way as other contaminants are being controlled today. This situation will likely arise out of the fact that while in the past it has been possible to modify disinfection practices to meet THM MCL's, in the future, this approach will not likely be possible. As a result, it will become necessary to focus THM control on significantly reducing THM precursor sources for domestic water supplies. For further information on this topic, consult Glaze (1991).
Role of Algae as THM Precursor Sources
It has been known for many years that the chlorination of laboratory algal extracts can lead to high concentrations of THM's. This has caused a number of investigators, principally Hoehn and his associates (Hoehn, et al., 1980) to investigate whether algae could be a significant source of THM precursors for domestic water supplies. Hoehn has found high concentrations of THM precursors in the presence of algal blooms in a reservoir in Virginia. Randtke, et al. (1988) conducted a series of studies specifically designed to examine the role that algae play in serving as THM precursors for the waterbodies that they investigated. They concluded that algae and other aquatic plants are not important sources of THM precursors in these waters. It appeared from their work, that while the algae and higher aquatic plants could serve as a THM precursor source, any precursors developed by or from them rapidly disappeared from the water.
It has been reported by Lee (1973) that the eutrophication of Lake Mendota located in Madison, Wisconsin that has occurred over the last 50 years or so has not changed the DOC of the lake water. At least for this waterbody, the DOC is primarily derived from terrestrial, land-based sources rather than aquatic plant, including algal, sources.
Walker (1983) has reported correlations between the phosphorus content of domestic water supply lakes and reservoirs and the THM's formed in these waters upon disinfection with chlorine. The implication is that since the phosphorus content of the lake correlates with algal chlorophyll, the algae are an important source of THM precursors. However, in the opinion of the authors, the correlation of phosphorus with THM's is spurious. It is likely that in many watersheds, phosphorus export from the land is correlated with DOC export from the land. Therefore, Walker's correlation approach cannot be judged as a valid assessment approach for determining the role that algae play as THM precursor sources.
From the information in the literature and the authors' experience, it appears now that it is important to distinguish between terrestrial and aquatic plants as THM precursor sources. While both terrestrial and aquatic plants can serve as important sources of THM precursors, it appears that the aquatic plant (algae and many macrophytes) produce THM precursors which are transitory-labile in aquatic systems. Terrestrial vegetation, on the other hand, tends to produce THM precursors, some of which are highly refractory-persistent in soils and aquatic systems. It has been suggested by Folan (1989) that this difference may be related to the lignin content of terrestrial plants. Lignin appears to be converted to highly persistent DOC. Since normally, aquatic plants have little or no lignin content, their decay, while initially producing large amounts of THM precursors, upon further microbial transformations, produce decay products which do not lead to THM formation.
While the literature on the persistence of algal-derived THM precursors is very limited, it appears to the authors that at least under warm water conditions of 15oC or greater the algal-derived THM precursors decay sufficiently in a few days to a week to non-precursor compounds. This decay would be expected to be somewhat slower in cold waters. There is obvious need to conduct in-depth studies on the formation and decay of algal-derived THM precursors in various types of aquatic systems of potential importance to water utilities. Such studies will provide utilities with the information they need to determine for their particular system whether THM precursors are derived at any time during the year to a significant extent from algal blooms in their raw water supply.
If a water utility finds, which is likely to be the case for water utilities with highly eutrophic raw water supplies, that algae represent a significant source of additional THM precursors, then there is additional justification for controlling algal populations through the use of nutrient (phosphorus and/or nitrogen) input control. Further, it may be appropriate for some utilities to develop pre-treatment of their raw water by biological means in order to bring about the decay of the algal-derived THM precursors before disinfection. This could be practiced by holding the water in the dark for a sufficient period of time to allow microbial transformation of the algal-derived THM precursors. It is likely that gentle stirring of the water such as with large paddles used in flocculation basins could accelerate the growth of bacteria which would bring about these transformations. It may be desirable to develop a modified version of a rotating biological contactor used for wastewater treatment as a means of developing sufficient bacterial populations for pre-treatment of the raw water. Such an approach would have a high probability of rapidly removing algal-derived precursors without stimulating additional algal growth or other raw water quality problems.
Water utilities that have high THM precursor concentrations in their raw water and have algal populations of greater than 10 to 20 ug/L planktonic algal chlorophyll in this raw water near the point of intake should determine if a significant part of the THM precursors are lost upon aeration and/or stirring of the water in the dark over a period of several days. If this occurs, then it may be possible to devise systems to accelerate the decomposition of labile precursors and thereby reduce the precursor load on the treatment works.
The Delta waters typically would be classified as moderately to highly eutrophic and would be expected to have a variety of algal related domestic water supply water quality problems, such as tastes and odors. It appears that the Contra Costa Water District and those whom this district supplies could expect that at least part of their THM precursor concentrations at some times in the year are derived from algae and therefore are potentially labile. This is an area that should be investigated since ultimately when the control of THM precursors from peat soils and other activities within the Delta is practiced, it could be that algae may become a very important part of the precursor sources for some of the water utilities drawing water from the Delta.
Management of Excessive Fertilization in the Delta
and in Water Supply Reservoirs
A review of the State of California Department of Water Resources Delta monitoring data for the period 1983 through 1989 shows that the amount of planktonic algal chlorophyll present during the period May through July at the Clifton Court Forebay averages about 7 to 25 ug/L. Many of the values are in the 10 to 20 ug/L range with some values exceeding 50 ug/L. As discussed below, algal growth within the Delta is about what would be expected based on the aquatic plant nutrients (phosphorus) available for their growth within the system. Based on the experience of the authors in relating planktonic algal chlorophyll to domestic water supply water quality problems, it is typically found that when the planktonic algal chlorophyll exceeds around 7 to 10 ug/L that water utilities can experience significant algal related water quality problems. It is well known (see Palmer, 1959) that algal related domestic water supply problems depend on the specific types of algae present. Some algae at planktonic algal chlorophyll concentrations in the 20 or so ug/L range cause few problems other than shortening filter runs. On the other hand, some algal blooms on the order of 5 to 10 ug/L chlorophyll cause severe taste and odor problems. There are situations, such as discussed above for Lake Tahoe, where taste and odor problems are found in water supplies in which the planktonic algal chlorophyll is on the order of 1 ug/L. Situations of this type appear to be very rare, however. There is general agreement that any time the planktonic algal chlorophyll concentration is above 25 ug/L, water utilities can expect to experience significant algal related water quality problems.
Based on the authors' discussions on algal growth within the Delta system with various individuals, it has been found that there is considerable confusion about how well the Delta grows algae compared to what it should be doing based on its nutrient loads and characteristics. It has been found by the authors that the amount of planktonic algal chlorophyll, as measured by the DWR Water Quality Surveillance Program from 1983 to 1989, at the Clifton Court sampling station for the period May through July is in reasonably good agreement with the amount of planktonic algal chlorophyll that would be expected at this location based on the phosphorus content of the water at that location. The predicted planktonic algal chlorophyll is on the order of 10 to 15 ug/L. The measured average values vary from 7 to 25 ug/L. The predicted values are based on predictions by the use of the Vollenweider-OECD modeling relationship discussed by Jones and Lee (1986). As discussed below, the Delta appears to have about a 30-day hydraulic residence time during the summer months, and therefore, there is ample time for algae to develop to the extent allowable based on the nutrients available.
It is clear from review of the DWR data that nitrogen is not the limiting element controlling algal growth in the Delta. There are significantly surplus amounts of nitrogen compared to what is needed to support the amount of algal growth that is occurring. Further, from the work of the authors (Jones and Lee, 1986) it is clear that light is not a significant limiting factor in controlling algal growth within the Delta over the control that light limitation has in controlling algal growth in other waterbodies, i.e., the color of Delta waters is not sufficient to significantly affect the biomass of algae that develops in these waters based on their nutrient content.
It is clear from these results that the Delta is growing algae in about the same way as waterbodies located throughout the world grow algae relative to their phosphorus loads. This is not unexpected since the stoichiometry (chemical composition) of algae is the same worldwide. The fact that some parts of California have a more arid climate does not, as is sometimes asserted, cause algae in this area to be different from algae in other areas of the world. It is also clear that water utilities that use Delta water as a raw water source can expect to have algal related water quality problems in their raw water supplies. It would be expected that water utilities using Delta waters would experience significant taste and odor problems and that there would be a potential for algal derived THM organic precursors in the water.
In addition to being concerned about algal derived tastes and odors and THM precursors for those utilities who take water directly from the Delta and treat it shortly after extraction, concern should also be focused on the development of algae in reservoirs that are used to store exported Delta water before its use as a domestic water supply source. Under these conditions, it is possible that severe algal related raw water quality problems could occur as a result of algae developing in the reservoir before the water is used for domestic purposes. Some water utilities, such as the Santa Clara Water District, have reported severe algal related water quality problems in waters derived from reservoirs that were filled with Delta water. This district has found a good correlation between planktonic algal chlorophyll and taste and odor problems in their raw water source. According to Means (1991), several of the Metropolitan Water District reservoirs, such as Perris Reservoir, have experienced significant algal related taste and odor problems. Other reservoirs in the Metropolitan Water District of Southern California (MWD) system have, on occasion, experienced similar problems.
Recently Karimi and Singer (1991) have reported that Silver Lake Reservoir, which is part of the Los Angeles Department of Water and Power (DWP) municipal water supply system, has significantly increased algal derived THM's. This situation arises from the chlorination of the reservoir water within the reservoir for the purposes of controlling algal growth. According to Heyer (1991), the restrictions on the use of Mono Lake tributary water as a water supply source for DWP has resulted in having to use water supplied by the MWD as a source. While the Mono Lake tributary water had low algal nutrients, the MWD water is derived from the Delta and has a high algal nutrient content. According to Heyer, coincident with the switch from Mono Lake tributary water to Delta water was an increase in the algal related water quality problems in some of the DWP reservoirs. Since the algae that are developing in some of these reservoirs, such as Silver Lake Reservoir, are not controllable by the addition of copper sulfate, this has caused DWP to initiate chlorination of the whole reservoir for the purpose of attempting to control algal growth. Karimi and Singer (1991) have found a correlation between the THM's in this reservoir water and the algae present in the water.
The Silver Lake Reservoir system is unusual because of the whole reservoir chlorination practice. Under these conditions, the THM precursors, which are algal excretory and degradation products and the algae themselves, are converted in the lake to THM's. It therefore becomes an issue of how fast the THM's present in the lake water dissipate rather than the dissipation of algal derived THM precursors discussed above.
The algal related water quality problems, including increased algal derived THM's, associated with the use of Delta water as a raw water source raises the question of whether it would be possible to control algal growth in the Delta as well as in off-Delta reservoirs filled all or in part with Delta water through the use of nutrient control at their sources for and within the Delta.
As discussed by Lee and Jones (1988a), there are approximately 50 million people in the world whose domestic wastewaters are being treated for phosphorus removal for control of algal related water quality problems in lakes and reservoirs. This is a well established technology typically involving the addition of alum (aluminum sulfate) as part of wastewater treatment to remove phosphorus by its incorporation into the alum floc. It is also possible to remove phosphorus through the use of biological uptake, precipitation with iron salts, or with lime. All of these methods are effective and widely practiced.
Ordinarily, for treatment works treating over one million gallons per day, the total cost of 90-95% phosphorus removal from domestic wastewaters is on the order of four cents per person per day contributing wastewater to the treatment plant. It is therefore appropriate to investigate whether phosphorus present in Delta waters used by water utilities as a raw water source is derived from readily controllable sources such as domestic wastewaters discharged to Delta tributaries or within the Delta.
In order to estimate whether phosphorus removed from domestic wastewater treatment plants which contribute phosphorus to the Delta via tributaries or directly, it is necessary to estimate the total phosphorus load that stimulates algal growth in the exported water. Since in normal precipitation years the high winter-spring precipitation runoff and snow melt flows from the Sacramento and San Joaquin rivers flush the Delta and since the algal related water quality problems associated with the use of Delta water are typically summer problems, the potential benefits for removing phosphorus from domestic wastewaters contributed to tributaries of the Delta should be evaluated for the summer.
It is estimated, based on DWR data from various sources, that the average residence time of water in the Delta during the summer months is about 30 days. This is based on an estimated volume of water in the Delta of 1 x 109 m3 and an estimated summer inflow of 15,000 cfs. It is, therefore, evident that during the summer there is ample time for algae to develop in the Delta to the extent possible from the nutrients (nitrogen and phosphorus) present in the river inflows to the Delta. Normally during summer months, about two weeks is necessary for algae to use all the nutrients they wish to use to develop peak biomass based on the characteristics of the waterbody.
It is possible that phosphorus added to the tributaries of the Delta during the fall, winter, and early spring could become important in causing algal related water quality problems during the following summer in a large reservoir that is filled with Delta waters principally derived from the Delta during the fall, winter, and spring. Under these conditions, consideration should be given to year round phosphorus removal from wastewaters and other sources should such removal be shown to have a potential benefit in reducing algal related water quality problems for utilities using waters from that reservoir.
Since 1983, the California Department of Water Resources (DWR) has been conducting an extensive monitoring program of Delta waters and its major tributaries (i.e. DWR, 1986 and other years). This monitoring program has included measurements of various nutrient species and planktonic algal chlorophyll. Based on review of this data, it is found that typically the concentrations of total phosphorus in the waters at the Clifton Court Forebay, where the waters are principally exported from the Delta, is on the order of 0.1 to 0.15 mg P/L. The typical tributary flow to the Delta during the summer months, according to various DWR documents, is on the order of 15,000 cfs. Using this flow and phosphorus concentrations, it is found that a total phosphorus load during the summer months of about 5 x 103 kg P/day is needed to account for the phosphorus present at the Clifton Court Forebay.
This approach assumes that all waters exported or discharged from the Delta are of the same composition as the waters at the Clifton Court Forebay. A review of the DWR data shows that the phosphorus content of the Sacramento River water near Point Sacramento and at Emmaton, both of which are just above where the main channel of the Sacramento River starts to mix with seawater, shows that the total phosphorus content of the water at this point is very similar to the phosphorus content at the Clifton Court Forebay during the summer months. Therefore, the assumption that all exported or discharged water from the Delta has a composition similar to the Clifton Court Forebay waters is reasonable.
Another approach to estimate the P load to the Delta is to determine the loads at Greene's Landing on the Sacramento River and Vernalis on the San Joaquin River. Using DWR phosphorus data for the summer at these locations and typical summer flows for these rivers, it is found that the estimated phosphorus load to the Delta is about 6 x 103 kg P/day. Therefore, the Clifton Court P load data and the Sacramento and San Joaquin River P load data at Greene's Landing and Vernalis, respectively, are in good agreement. It therefore appears that, at least over the summer period, the processes that take place in the Delta that remove or add phosphorus to the water tend to balance out where the phosphorus load input into the Delta is approximately equal to the phosphorus load exported and discharged from the Delta.
According to Rast and Lee (1983), the typical phosphorus per capita contribution to domestic wastewaters in the US is about 1 kg P/year. According to DWR Bulletin 160 in 1987, the Sacramento River basin had about 1.87 million people and the San Joaquin River basin had about 1.18 million people. Therefore, in these two river basins there are about 3 million people that could be contributing phosphorus to domestic wastewaters that ultimately enter tributaries of the Delta. In addition, there are about 1.3 million people in the Tulare Lake basin. However, in many years, the Tulare Lake basin does not contribute water to the Delta system. For the purposes of this review, it is assumed that the 1.3 million people in the Tulare Lake basin do not contribute phosphorus to the Delta during the summer months. It will also be assumed that between 2.5 to 3 million people in the Sacramento and San Joaquin River watersheds contribute phosphorus to the rivers or to tributaries of these rivers and ultimately into the Delta. Based on this approach about 7 x 103 kg P/day could be contributed to the Delta from domestic wastewater sources. According to Archibald (1991), the average estimated domestic wastewater flows to tributaries of the Delta is about 260 mgd (million gallons per day). Using 2.5 x 106 people as an estimate of the population contributing wastewaters to the Delta tributaries and an estimated per capita flow of about 100 gpd (gallons per day), it is found that there is good agreement between the estimated domestic wastewater flow and the average measured domestic wastewater flow.
The drainage basin for the Delta is shown in Figure 4. According to WRCB (1990), the Sacramento River drains 16,960,000 acres, the Central Sierra area drains 2,432,000 acres, and the San Joaquin River drains 7,040,000 acres. Therefore, there are approximately 26 million acres that can contribute phosphorus to the Delta from land runoff above the Delta. As reported by Rast and Lee (1984) (see Table 3), typically forested and agricultural lands contribute from 0.005 to 0.05 g P/m2/yr. If it is assumed that the export of phosphorus from land in the Delta drainage basin is 0.01 g P/m2/yr, it is estimated that about 3 x 102 kg P/day could be contributed by land runoff to the Delta tributaries. This approach assumes that the amount of phosphorus contributed from land runoff is equally partitioned for each day over the year. It is well known that this is not the case. Phosphorus contributed from land runoff typically occurs during the high runoff period in the late winter, early spring. It would be expected that except for some agricultural drainage that most of the lands in the tributaries of the Delta would contribute very little phosphorus to these tributaries in the summer months.
Another factor that would tend to make the estimated phosphorus loads from land runoff high is the fact that many of the headwaters of these tributaries contain reservoirs. Reservoirs tend to be efficient traps for phosphorus. Ordinarily, on the order of 80% of the phosphorus entering a reservoir is trapped within the reservoir and becomes part of the reservoir sediments. It is therefore likely that a large part of the phosphorus that would be derived from agricultural runoff above the reservoirs would not be transported to the Delta.
In addition to phosphorus contributed to the Delta tributaries from land runoff and domestic wastewater sources, consideration should be given to phosphorus sources within the Delta. There are two principal sources of phosphorus within the Delta. One of these is wastewater discharges to Delta channel waters and the other is drainage from the agricultural lands within the Delta. According to DWR (1989), there are approximately 200,000 people living in the Delta system. If all of the phosphorus in the domestic wastewaters from these people were discharged to the Delta channels, it would represent an insignificant additional source of phosphorus for the Delta. It appears, however, that a very small fraction of the wastewaters associated with this population are discharged to Delta channels that could represent a source of phosphorus for the waters exported from the Delta in the State Water Project. According to Archibald (1991), approximately 14,500 people living within the Delta discharge wastewaters to the Delta. It is therefore concluded that domestic wastewater sources of phosphorus for the Delta are insignificant sources of phosphorus for the Delta.
According to DWR (1989), there are about 520,000 acres of agricultural land within the Delta. These lands are fertilized for agricultural crop production. It would be expected that part of this fertilizer would be present in the agricultural drains from the Delta islands. If it is assumed that the phosphorus export coefficients from the Delta island agricultural activities is 0.1 g P/m2/yr (a high value for most agriculture), it is found that the Delta island agricultural activities could potentially contribute on the order of 1 x 103 kg P/day to Delta channel waters.
Agee (1991) provided the authors with some DWR monitoring data for the phosphorus content of agricultural drains from Empire Island within the Delta. This data covered about 2.5 years of sampling during the period 1987-89. While the phosphorus concentration values in the drainage water were highly variable, the average of the 30 values is 0.13 mg P/L. It is therefore evident that, at least for Empire Island, the amount of phosphorus in the agricultural drainage water is about the same as the phosphorus diverted from the channels to this island. Therefore, since the load of phosphorus exported at the Clifton Court Forebay and discharged from the main stem of the Sacramento River to the San Francisco Bay system is approximately equal to the amount of phosphorus contributed to the Delta at Greene's Landing and Vernalis on the Sacramento and San Joaquin rivers, respectively, and since there are no obvious potentially large sources of phosphorus within the Delta other than agricultural drainage and since the agricultural drainage data does not show high phosphorus content compared to the Delta channel waters, it is concluded that phosphorus sources within the Delta are insignificant compared to phosphorus sources in the tributaries to the Delta.
It is, therefore, evident that the amount of phosphorus contributed from land runoff to the Delta tributaries during the summer months is insignificant compared to the amount of phosphorus derived from domestic wastewater sources which are discharged to the tributaries of the Delta. While these estimates are based on general overall characteristics of the Delta and its tributaries, it is clear that a substantial part of the summer phosphorus load to the Delta could be derived from domestic wastewaters discharged to tributaries of the Delta. These estimates indicate that domestic wastewater sources of phosphorus for the Delta could be a significant part of the total P load. Therefore, it is appropriate to pursue refining the estimates of the potential benefits of controlling phosphorus in domestic wastewaters on algal related water quality problems for water utilities that use Delta water as a raw water source. The authors are in the process of obtaining additional data that could be used for this purpose.
As discussed by Jones and Lee (1986), it is important to evaluate whether at least 25% of the total P load for a particular waterbody is controllable in order to ascertain whether phosphorus control programs would likely produce some benefit in reduced algal biomass. It is now well established that at least this amount of phosphorus must be removed in order to cause a discernible change in algal biomass. It is highly inappropriate to assert, as has been done by those not familiar with the results of eutrophication management programs, that in order to produce an improvement in eutrophication related water quality, it is necessary to reduce the planktonic algal chlorophyll to less than about 5 ug/L. It is well known from actual experience in many waterbodies where phosphorus input control has been practiced that significant benefits in both recreational and domestic water supply water quality have been found whenever on the order of 25% or so of the total available phosphorus load is controlled. The improvements in water quality occur independent of the trophic state (chlorophyll concentration) of the waterbody. The 5 ug/L chlorophyll level value is based solely on improving the algal related water clarity (Secchi depth) for recreational use and has little or nothing to do with domestic water supply raw water quality or, for that matter, many of the other recreational impacts of eutrophication such as the frequency and severity of obnoxious algal blooms that occur in a waterbody.
It is important in making the evaluation of P loads to the Delta to focus on the control of those loads that lead to algal available P in the waterbodies where there is concern about algal impacts on domestic water supply water quality. As discussed by Lee et al. (1980), there are a variety of chemical and biological processes that take place in aquatic systems that convert algal available forms of phosphorus into non-available forms and vice versa. Typically, however, in rivers and in aquatic systems like the Delta the net conversion would likely be toward forms not available to support algal growth. It would therefore be important to conduct in-depth studies of the aqueous environmental chemistry of phosphorus in the tributaries to the Delta, within the Delta, the water export systems from the Delta, and within any off-Delta reservoirs in order to focus the phosphorus control programs on those parts of the phosphorus which are responsible for stimulating algal growth. Well established methodologies are available today to determine algal available phosphorus. For further information on this topic, consult Lee et al. (1980).
An additional source of phosphorus for domestic water supply reservoirs in the central and southern part of the state is the irrigation return water that enters the aqueduct system that transports water to the south and directly into some reservoirs that are part of this system. At this time the authors do not have data on the phosphorus content of the waters entering various reservoirs in the southern part of the state where algal related water quality problems have been found. If such data does not now exist, it should be developed in order to ascertain whether there are significant sources of algal available phosphorus that could stimulate algal growth in reservoirs in the southern part of the state. If significant sources of this type exist, then phosphorus control programs should be considered for these sources. The direct addition of alum to these waters may be a highly cost effective way of removing phosphorus from sources of this type (see Lee, 1973).
According to Means (1991), significant algal populations are found in the aqueduct system transporting Delta waters to the south. As part of developing algal control programs, consideration should be given to the role that algae that develop in the aqueduct play in causing algal related water quality problems to the water utilities that use aqueduct waters as a source.
It is important to understand that the frequently used approaches for estimating whether nitrogen or phosphorus is limiting algal growth in a lake or reservoir are often inappropriate. Attempts to look at total phosphorus/nitrate ratios for estimating nutrient limitations are unreliable for estimating the impact of altering phosphorus loads to a waterbody on the planktonic algal growth that occur within the waterbody. As discussed by Lee and Jones (1981) in an AWWA Quality Control in Reservoirs Committee report, in order for nitrogen or phosphorus to limit the biomass of algae that develops in a waterbody, the concentrations of available forms must be below growth rate limiting concentrations at peak biomass when there is concern about algal related water quality problems. Ratios of nutrients are unreliable predictors of algal limiting nutrients and can readily lead to erroneous conclusions about the potential benefits of controlling nitrogen or phosphorus inputs to a waterbody on reducing algal related water quality problems.
Rast et al. (1983) have shown that even though the growth rate of algae in a waterbody is not controlled by phosphorus, it is possible to use the Vollenweider-OECD modeling relationships described by Jones and Lee (1986) to predict the potential benefits of controlling phosphorus input to a certain degree on the algal related water quality of a waterbody. As discussed by Jones and Lee (1986) and Rast et al. (1983), the Vollenweider-OECD and post-OECD database, which now exceeds over 500 waterbodies located in various parts of the world (see Figure 5), shows that changing the phosphorus load to a waterbody produces in most waterbodies a readily predictable change in the planktonic algal chlorophyll concentration that developed in the summer within the waterbody. This relationship holds even though phosphorus is not an algal growth rate limiting element in the waterbody, i.e., phosphorus is surplus compared to algal needs. This appears to be the case throughout the Delta system and in down-Delta reservoirs.
Figure 5 shows that there is a relationship between the normalized phosphorus loads to a waterbody and the planktonic algal chlorophyll that develops within the waterbody. The normalizing factors are the waterbody's mean depth and hydraulic residence time. The abscissa term in Figure 5 includes L(P) which is equal to the areal annual P load in mg P/m2/yr) divided by the qs which is the mean depth divided by the hydraulic residence time (Tw in years) in m/yr. The mean depth of the waterbody is the volume of the waterbody divided by its surface area. The hydraulic residence time is the volume of the waterbody divided by the annual inflow rate. The abscissa normalizing term has been found to be approximately equal to the annual phosphorus concentration in the waterbody. Therefore, the relationship shown in Figure 5, in its most basic terms, is simply a statement of algal stoichiometry in which there is a correlation between the phosphorus concentration in a waterbody and the algal growth that occurs in the waterbody. While this relationship is not applicable to all waterbodies, it is applicable to well over 80% of the world's freshwater waterbodies. Jones and Lee (1986) provide guidance on how to determine its applicability to a particular waterbody.
In order for the growth of algae in a waterbody to be proportional to the available phosphorus concentrations in the water, even though phosphorus is not limiting their rate of growth, it is necessary that all other nutrients needed by the algae be present in surplus amounts compared to algal needs. The chemical of typical concern in this regard is nitrogen in the form of nitrate and/or ammonia. The DWR monitoring data for the Delta waters shows that nitrogen is not limiting algal growth in these waters. Further, since algal growth in the Delta is about equal to what is predicted based on phosphorus chlorophyll relationships for waterbodies located throughout the world, it appears that all other elements needed for algal growth are present in sufficient concentrations to allow growth to the extent possible based on the characteristics of the Delta and the phosphorus loads.
From the information available at this time, it appears that phosphorus should be added to the list of contaminants of Delta system waters that should be investigated for the possible development of control programs. There is a potential for such programs to significantly improve the algal related tastes and odors and other domestic water supply water quality problems, including THM precursor formation, through phosphorus control in the Delta system and its tributaries. Such control programs could affect domestic water supply water quality for many millions of people in California.
One of the potential consequences of phosphorus control for tributaries of the Delta and in the Delta is the decreased fish production within the Delta. Jones and Lee (1986) have reported a strong, highly significant relationship between the phosphorus loads to waterbodies located in various parts of the world and the fish production within these waterbodies (see Figure 6). Basically, the relationship is one of increased primary production (algae) in lakes and reservoirs resulting in increased secondary (zooplankton) and tertiary (fish) production. Since the primary productivity and algal biomass in many lakes and reservoirs, as well as other waterbodies, is correlated with the phosphorus concentration within the waterbody and since phosphorus concentrations within the waterbody can be correlated with phosphorus loads when normalized by the waterbodies' hydrological and morphological characteristics, it is not surprising that a relationship is found between normalized phosphorus loads in lakes and reservoirs located in various parts of the world and fish production. Therefore, decreasing the phosphorus loads to the Delta will likely decrease the fish production within the Delta.
Using the relationship developed by Jones and Lee (1986), between normalized phosphorus loads and fish production, it is found that in the range of planktonic algal chlorophylls of concern within the Delta system that a 50% reduction in the phosphorus load to the Delta would be expected to decrease fish production by 40 to 60% dependent upon the planktonic algal chlorophyll concentration. While there may be some who assert that decreasing phosphorus loads to the Delta system should not be practiced because of the adverse effects on the fisheries of the Delta, it is clear that the problems of the fisheries of the Delta are not fish food supply related and therefore controlling phosphorus inputs will likely have little or no impact on fish production for the fish species of primary concern in the Delta, such as striped bass. Phosphorus control, however, will almost certainly have an impact on the rough fish population, such as carp.
It is, therefore, concluded that because of the importance of the Delta as a water supply source for two-thirds of the population of California that a much greater effort should be devoted to source water quality control for contaminants that either directly or indirectly, as in the case of phosphorus, cause significant water quality problems for water utilities that use Delta waters as a source of supply. Understanding the specific sources of various contaminants and investigating the potential for control of these contaminants at the source could be significantly beneficial in improving domestic water supply water quality for many of the people in California.
Management of Eutrophication
Lee and Jones, through their activities in the AWWA Quality Control in Reservoirs Committee, developed a report that was reviewed and approved by the committee which serves as a guide to water utilities on the approaches that should be considered in evaluating whether phosphorus control from watershed sources could be a potential benefit in improving a water utility's domestic water supply raw water quality. Additional information on this topic is provided by Lee and Jones (1984a, 1988b) and Jones and Lee (1986). An example of the application of the evaluation of the potential benefits in controlling phosphorus loads to a domestic water supply reservoir is provided for Lake Ray Hubbard, a city of Dallas, Texas water supply reservoir, by Archibald and Lee (1981).
There are a variety of techniques that have been used with success in some locations for management of eutrophication of waterbodies. Generally, the utility of these approaches has been judged based on improvement of recreational uses of the water. Thus far, inadequate attention has been given to the improvement of domestic water supply raw water quality. A review of the various techniques that have been used for managing eutrophication has been published by Lee (1973) and by Cooke et al. (1986).
While there are several techniques, such as aeration, dredging, manipulation of fish and other aquatic organism populations, aquatic weed harvesting, etc., that have been used with some success for managing eutrophication related recreational impacts in lakes and reservoirs, it is questionable whether many of these techniques have applicability to significantly improving domestic water supply eutrophication related water quality. For example, one of the techniques that is often said to be beneficial for managing eutrophication related water quality in lakes and reservoirs is aeration-destratification of the waterbody. This technique, however, does not necessarily improve eutrophication related water quality for recreational and domestic water supply uses.
The value of aeration of reservoirs in improving domestic water supply water quality was reviewed by the AWWA Quality Control in Reservoirs Committee. This committee reported that after extensive review of the data available, there were serious questions as to whether aeration of a water supply reservoir would improve water quality. It was found that in some water supply reservoirs, aeration caused greater algal related water quality problems than occurred in the unaerated reservoirs. This situation is to be expected in stratified reservoirs where the thermocline serves as an effective barrier to nutrient regeneration and transport from the deeper waters of the lake to the surface waters where the algae develop. The aeration-destratification of a water supply reservoir, however, should be evaluated cautiously. It appears that in some instances, but not all, there are benefits in domestic water supply water quality associated with aeration-destratification of the waterbody. As discussed by Lee (1973), hypolimnetic aeration of reservoirs in which destratification does not occur has been found to be an effective method of improving the domestic water supply water quality of hypolimnetic waters.
It is important for water utilities that are facing eutrophication related water quality problems to focus their efforts to the greatest extent possible on controlling algal nutrients. Efforts to control eutrophication by other methods must be carefully evaluated.
Control of Hazardous and Other Chemicals
Typically, water utilities and regulatory agencies conduct fairly effective programs for control of hazardous contaminants, such as heavy metals, pesticides, etc., that can cause significant water quality problems in domestic water supply. Usually, such problems are detected through the routine monitoring that is done by the utility and regulatory agencies. When excessive concentrations of a contaminant are found, it is usually relatively straightforward to develop control programs for that contaminant from the particular source(s). It is important to point out, however, that the routine monitoring programs that are typically conducted by water utilities and regulatory agencies measure only a small number of the potentially significant chemicals that can be present in an urbanized-industrialized watershed. While water pollution, air pollution, and solid and hazardous waste management programs are becoming more effective in controlling the discharge of known, highly hazardous chemicals, such as the priority pollutants, water utilities should go beyond the routine monitoring to critically evaluate whether there are other sources of chemicals in their watershed that could degrade domestic water supply water quality. Basically, water utilities should become highly pro-active toward protection of their water supply sources from all chemicals that could be adverse to providing a potable and palatable water.
Groundwater Quality Protection
Many water utilities, especially in California, have all or parts of their domestic water supply based on groundwater sources. Some communities, such as Pittsburg, California, even though they have 100% of their normal domestic supply provided by surface water sources, have installed standby well(s) as an emergency supply during drought or other conditions which would interrupt the surface supply source. The current drought has emphasized the importance of a highly developed, coordinated conjunctive use program in the state of California, where during wet years, surplus surface water is recharged into groundwater basins. This recharged water would then be available for use during future droughts. This drought has also pointed to the extreme importance of protecting groundwater and groundwater aquifer quality. For many years, the state of California has had regulations which prohibit activities that can lead to groundwater pollution. It is the experience of the authors, however, that over the years, including today, these regulations are not being adequately implemented with the result that groundwater pollution is still occurring at a significant rate in various parts of the state. In many cases, such as those associated with municipal and some industrial solid waste disposal by land burial, the groundwater pollution is not only destroying the use of the water for domestic and some other purposes, but is also destroying the use of parts of the aquifer for conjunctive use storage. It is therefore important that every possible step be taken to protect groundwater aquifer systems from immediate as well as long-term pollution.
One of the most potentially significant sources of groundwater pollution for waters that could be used for domestic water supply purposes is by municipal landfill leachate. US EPA estimates that there are on the order of 75,000 landfills in the US with over 75% of them polluting groundwaters at this time. In California, the regional and State Water Resources Control Boards as part of their Solid Waste Assessment Test Annual Report to the legislature concluded that of the approximately 300 landfills in the state investigated thus far, over 80% of them are polluting groundwaters. While existing groundwater pollution by municipal and industrial landfills is occurring from unlined landfills, the clay and membrane lined landfills of the type being constructed today ("dry tombs") are widely recognized as simply postponing the problems of groundwater pollution by landfill leachate. Ultimately, as discussed by Lee and Jones (1991), the landfill cover will fail to keep moisture out of the landfill and the landfill liners will fail to keep leachate from polluting groundwaters.
Table 4 presents information on the typical composition of municipal landfill leachate for the common contaminants. Typically municipal landfill leachate must be diluted at least a thousand-fold and more commonly over ten-thousand-fold before groundwaters contaminated by such leachate would be considered to comply with drinking water standards (MCL's) from known leachate constituents. Since very limited dilution of contaminants occurs in groundwater, it is evident that municipal landfill leachate represents a highly significant threat to domestic water supply water quality. The US EPA (1988) has determined that when a groundwater well is contaminated by municipal landfill leachate that it is appropriate to assume that the well has to be abandoned and a new well be constructed in a different aquifer or at a sufficiently distant location so that it will not intercept any groundwaters contaminated by leachate. This situation arises from the fact that contaminants in municipal landfill leachate are of such a nature as to make it impossible to be removed from the aquifer to a sufficient degree to render the aquifer waters usable for domestic purposes. While in many parts of the country construction of new wells to replace those that have been contaminated by leachate is feasible, in the more arid areas and ultimately everywhere this approach cannot be followed since there is a finite amount of groundwater available that can be used for domestic water supply purposes.
Table 4: Typical Nutrient Loads to Lakes and Reservoirs
|
Land Use |
Total P (g/m^2/yr) |
Total N (g/m^2/yr) |
|
Urban |
0.1 |
0.25 |
|
Rural/Agriculture |
0.05 |
0.2 |
|
Forest |
0.005 |
0.1 |
|
Atmosphere |
0.025 |
1.0 |
[After Rast and Lee, 1982]
It is important to understand the difference between the pollution of domestic water supply groundwaters by VOC's, such as TCE, and by municipal landfill leachate. While it is relatively easy to remove many of the VOC's from contaminated groundwaters and produce a water that is considered suitable for domestic consumption, it is extremely difficult if not impossible to treat a groundwater contaminated by municipal landfill leachate to the degree necessary so that it would be considered appropriate for domestic water supply use. Municipal landfill leachate contains a wide variety of contaminants which are highly difficult to remove. Further, because of the large amounts of uncharacterized, unknown, non-conventional contaminants in landfill leachate, using treated groundwaters for domestic water supplies that have been contaminated by municipal landfill leachate will always be a threat to public health since it will never be possible to be certain that the treatment has removed all hazardous chemicals.
Another consequence of contaminating groundwaters by municipal landfill leachate which is of major significance to some water utilities is the loss of aquifer storage capacity as part of conjunctive use of surface and groundwaters. Those parts of aquifers that have been contaminated by municipal landfill leachate cannot be used for domestic water supply conjunctive use even though attempts are made to try to flush out the residual contaminants in the aquifer. It is therefore apparent that domestic water supply utilities and, for that matter, individual homeowners who depend on groundwaters near a municipal landfill must be highly concerned about the potential for groundwater contamination by landfill leachate.
Presented below is a suggested set of actions that municipal water agencies and water districts should take to protect the quality of existing and potential groundwater water supply sources from landfill contamination.
1. Determine if existing and previously closed sanitary landfills or other waste management units are contaminating groundwaters.
Any groundwater contamination by municipal landfill leachate, independent of whether it causes a drinking water standard to be exceeded, should be considered to be a serious threat to public health and domestic water supply water quality. Generally, state water pollution control agencies are requiring that all landfill owner/operators establish groundwater monitoring programs for active as well as closed landfills. Water utilities should periodically review the state and/or local agency files to determine the adequacy of the groundwater monitoring programs that have been established for the landfills located in their aquifer recharge area. This should be done by an individual on the utility's staff or a consultant who is highly familiar with groundwater quality monitoring near landfills.
It is the authors' experience that typically the groundwater monitoring programs that are being required by state agencies for existing, much less previously closed, landfills are inadequate to define with a high degree of certainty whether pollution of groundwater is occurring and the degree and extent of pollution. It may be necessary for the utility to request and, if necessary, take legal action, to require that the state and/or local agency responsible for groundwater quality protection will require that the owner/operator of existing as well as previously closed landfills establish an adequate groundwater monitoring program for each landfill that could contaminate the utility's aquifer. It is suggested that the groundwater monitoring programs be designed so that they would have at least a 95% probability of detecting groundwater pollution by landfill leachate. As discussed by Lee and Jones (1991), this will require a much more extensive groundwater monitoring program than is typically being developed today for landfills.
2. If contamination of an aquifer that is or could be used for domestic water supply purposes has occurred, require that the owner/operator of the landfill define the areal extent and degree of groundwater contamination by the landfill.
The determination of the extent and degree of groundwater contamination by a landfill will require that an extensive set of monitoring wells, typically nested to sample water at various depths at various locations, be used. These wells should be sampled at no less than quarterly intervals over one year to define the degree and extent of contamination that has occurred. Normally, such a sampling program has to be conducted in phases where at the end of the first phase, when it becomes clear that insufficient information is available to fully define the extent and degree of contamination, that additional monitoring wells will have to be constructed and sampled.
3. Require that the landfill owner/operator initiate comprehensive groundwater quality remediation programs to try to remove all contamination from the groundwater and the aquifer.
Work on remediation of Superfund sites is now showing that groundwater remediation from simple contamination, such as from VOC's, is difficult to achieve. It is clear now that typically it will take many tens of years of continuous pumping of the contaminated water in order to stop the spread of the contamination and to reduce the degree of contamination to the maximum extent possible. It is becoming recognized that for some types of contaminants it may not be possible to achieve background concentrations. The owner/operator of the landfill, however, should be required to aggressively pursue a remediation program to achieve background concentrations of contaminants to the maximum extent possible.
4. If the owner/operator of an active landfill cannot prevent further contamination from the landfill, the owner/operator should stop accepting wastes and close the landfill. If closure does not stop groundwater contamination, require that the waste be exhumed and properly treated, and the residues be deposited at a suitable location where groundwater pollution will not occur.
It has become clear that in many instances the only way to truly protect a domestic groundwater supply from municipal landfill leachate contamination is to exhume the wastes. This will be especially true for those landfills that are located in areas where moisture can enter the landfill from groundwaters. In situations where the only source of moisture for leachate generation is through the cap, it may be possible to stop further groundwater pollution by leachate generated in the landfill by construction of a cap that will, in fact, prevent moisture from entering the landfill. It is important to note that the typical landfill caps that are being constructed today are inadequate to prevent leachate generation within the landfill and will not achieve this objective. Further, the owner/operator of a landfill that is capped as a means of attempting to prevent groundwater contamination must be required to maintain the cap for as long as the wastes are present (forever) in order to prevent moisture from entering the landfill. It is felt that any owner/operator that fails to provide this type of maintenance of the cap must be required to exhume the wastes.
5. For all landfills that could effect a domestic groundwater supply, the water utility should require that all groundwater quality monitoring data on the landfill be sent to the utility for the utility's review and comment at the time that it is submitted by the landfill owner/operator to the regulatory agencies.
For existing as well as closed landfills, water utilities should take a pro-active approach to groundwater quality protection where they have specific staff members or consultants who will review all routine groundwater monitoring data as it is submitted to the agency by the owner/operator of the landfill. The authors have seen situations where the regulatory agency personnel do not have time or do not appreciate the significance of the potential damage that municipal landfill leachate can cause to a groundwater based domestic water supply. They also may not understand the importance of detecting leakage from a landfill at the earliest possible time. It is therefore imperative that the water utilities conduct their own independent data review of the groundwater quality monitoring program at all active as well as closed landfills that could contaminate the aquifer.
6. Water utilities should require that all owners/operators of landfills that could impact existing or potential domestic water supplies maintain the leachate removal system, the groundwater monitoring system, the landfill cap, and all groundwater diversion systems FOREVER.
Water utilities should review the financial assurance instruments that are submitted by owners/operators of landfills that are designed to provide for post-closure monitoring and maintenance of the landfill. At this time, in some states, no financial assurance is required for municipal landfill post-closure operations. In others, such as California, the financial instruments used by owners/operators of landfills are grossly inadequate to provide the amount of funds necessary to provide for required post-closure care activities that will prevent the landfill from polluting groundwaters at any time in the future. Water utilities should aggressively work toward requiring that the owner/operator of the landfill and the regulatory agencies establish a trust fund that will ensure that adequate funds are available to carry out these activities FOREVER.
For active landfills, the trust fund can be developed from disposal fees. For previously closed municipal landfills that could contaminate a domestic water supply, the utilities should work through the state and local agencies and, if necessary, the courts to require the principle responsible parties who owned/operated the landfill, as well as the public that contributed waste to the landfill, to develop a trust fund of sufficient magnitude to ensure that the landfill will be properly closed and maintained FOREVER. The magnitude of the trust fund should be sufficient to cover not only the post-closure monitoring and maintenance but also the costs to exhume the wastes, properly treat them, and rebury the non-recyclable, non-reusable residues at an appropriate location that will not contaminate groundwaters in the future.
7. Water utilities should aggressively pursue developing approaches for the management of solid waste in their groundwater supply watersheds that will minimize the potential for groundwater quality problems at any time in the future. They should oppose the "dry tomb" approach for municipal solid waste management in areas where domestic water supplies could be contaminated because of the high probability that that approach will ultimately lead to groundwater contamination.
It is suggested that it would be appropriate for water utilities to require that the owner/operator of existing as well as proposed landfills provide a detailed discussion of the plausible worst case scenarios that could occur at the landfill that could lead to groundwater pollution. The reports made by consulting firms working on behalf of governmental agencies and/or landfill owners/operators in the environmental impact statements (EIS's) or in California environmental impact reports (EIR's) typically do not properly assess the potential for groundwater pollution by landfills. During the past couple of years the authors have frequently observed consulting firms working on behalf of the applicant for a landfill make such statements as "since the landfill is lined, there can be no water pollution." Another example is that "any leakage of leachate from the landfill will be detected by the groundwater monitoring system. Once detected, remediation programs will be initiated which will clean up the groundwaters." Such statements are not an appropriate assessment of the current understanding of the ability of landfill liners to prevent groundwater pollution and groundwater monitoring systems to detect it once it has occurred. Further, as discussed above, it is not possible to completely clean up an aquifer contaminated by municipal landfill leachate.
As part of evaluating the worst case scenario(s) for groundwater pollution by a landfill, the owner/operator of a landfill should be required to provide detailed discussion of how they will prevent groundwater pollution at a particular landfill based on worst case scenario conditions. They should also provide detailed discussions with associated cost estimates of what remediation steps they will take to remediate the groundwaters that are polluted by landfill leachate. The worst case scenario should consider that the proposed groundwater monitoring program will fail to detect groundwater pollution. It should be assumed that a pollution plume has occurred for considerable distances downgradient where it is detected in production wells used for domestic water supply or other purposes.
One of the best ways for water utilities to protect their groundwater supplies from pollution by new landfills is to develop a highly aggressive program of work toward developing alternative methods of managing municipal solid and industrial wastes so that they are not buried in "dry tombs" where they can ultimately pollute groundwater. It is clear that the "dry tomb" approach is not a viable approach for municipal solid waste management in most parts of the US. Alternative approaches are available. While initially more expensive compared to what the public has become used to paying for municipal solid waste disposal, in the long-term they will be less expensive and provide for true long-term groundwater quality protection. For additional information on the potential significance of pollution of groundwaters by municipal landfill leachate, consult Lee and Jones (1984b, 1991).
While the focus of this groundwater quality protection program is municipal landfills, similar kinds of programs should be directed toward all waste management units, such as wastewater lagoons, as well as agricultural uses of chemicals. Further, utilities with groundwater supplies near saline waters, such as along the coast, should be determining whether saltwater intrusion is occurring to a significant extent that could ultimately pollute the groundwaters of the region.
It is the experience of the authors that inadequate attention is being given to the potential for groundwater contamination by chemicals in surface waters that are deliberately recharged or that naturally recharge aquifers. Water utilities should be conducting intensive monitoring programs of all recharged waters to ensure that such waters do not contain contaminants that will pollute the aquifer.
Conclusions
It is evident that there are a number of ways in which municipal water agencies-utilities and regulatory agencies can improve domestic water supply raw water quality by implementing pollutant control programs at the source. It is well known that eutrophication related water quality problems are controllable through the use of algicides, such as copper sulfate, or through reductions in the amount of aquatic plant nutrients, especially phosphorus, entering a lake or reservoir.
Municipal water utilities should be evaluating the activities that take place in the domestic water supply watershed that could be adverse to their raw water quality. In addition to problems associated with algal growth and the limitation of phosphorus inputs to lakes and reservoirs, water utility watershed activity, concern should be focused on evaluating potential sources of all chemicals and microbial contamination that could cause water supply water quality problems. While water utilities have a long history of aggressively pursuing the control of industrially and agriculturally derived contaminants, such as phenols, toxic chemicals, pesticides, etc., in general, water utilities have not been sufficiently aggressive in controlling nutrients that lead to excessive fertilization related water supply water quality problems.
With increased attention being given to control of THM's in treated waters, emphasis should be placed on understanding the sources of organic THM precursors. A significant effort should be made to develop THM precursor land use export coefficients. Each water utility should determine the dominant sources of THM precursors in its watershed and evaluate on a site-specific basis the potential for control of the most significant sources. THM precursor control programs should be initiated in those situations where the collective development of such a control program would result in a significant lowering of the THM's produced upon disinfection of the water supply.
Those water utilities that have significant sources of bromide within their watershed should aggressively pursue controlling the bromide at its source in order to prevent, or at least minimize, brominated THM formation.
Water utilities utilizing groundwaters as all or part of their supply, at this time or potentially in the future, should adopt a highly pro-active program of groundwater quality protection from municipal and industrial landfills, waste treatment lagoons, agricultural chemical use, subdivisions employing septic tank wastewater disposal systems, etc. Included within this program should be a careful monitoring of the quality of all waters that are recharged to groundwater as part of a conjunctive use program as well as recharge that occurs naturally. This program should be designed to prevent further groundwater pollution which would not only destroy the use of groundwaters for domestic purposes, but would also impair the use of the aquifer for conjunctive use storage.
Municipal water utilities and agencies that use the Sacramento-San Joaquin River Delta as a water supply source should investigate the potential benefits of the control of phosphorus in domestic wastewater sources discharged to tributaries of the Delta. It has been found that during the summer months, domestic wastewater sources are the primary source of phosphorus for the Delta system. Phosphorus control from these sources with readily available, widely practiced technology could result in a significant reduction of algal growth within the Delta and in down-Delta reservoirs as well as in the aqueduct system. Such reduced growth could significantly reduce the algal related taste and odor problems as well as algal derived THM precursors.
The algal related taste and odor problems that have begun to occur in Lake Tahoe appear to be related to increased planktonic algal growth in the open waters of the lake and especially increased periphyton (attached algal) growth in the nearshore waters. These increased growths are related to increased nitrogen input to the lake from atmospheric sources and nitrogen and phosphorus input to the nearshore waters of the lake due to groundwater transport of fertilizers used for lawn and shrubbery fertilization. In order to reduce the frequency and severity of algal related domestic water supply water quality problems in Lake Tahoe, it will be necessary to significantly curtail the use of automobiles and other vehicles powered by internal combustion engines in the Lake Tahoe watershed and to ban the use of lawn fertilizers and lawns within the lake's watershed.
Acknowledgements
The authors wish to acknowledge the assistance provided by several individuals in developing this paper. Numerous members of the California Department of Water Resources were helpful in providing information. Of particular significance was Dr. S. Hayes, B. Agee, R. Woodard, and R. Zettlemeyer. The assistance of Dr. D. Carlson of the Water Resources Control Board staff in obtaining background information for this paper is also acknowledged. W. Heyer and Dr. A. Kamini of the Los Angeles Division of Water and Power, E. Means and R. Clemmer of the Metropolitan Water District of Southern California, and several individuals with the Sacramento Office of the US Geological Survey provided important background information for this paper. Special recognition should be given to L. Hoag, Executive Director of the California Urban Water Agencies and to H. Vaux, Director of the University of California Water Resources Center.
Figure 5: Relationship between Normalized P Loading and Chlorophyll in Lakes and Reservoirs World-Wide [After Jones and Lee, 1986]

Figure 6: Relationship between Normalized P Loading and Fish Yield [After Jones and Lee, 1986]
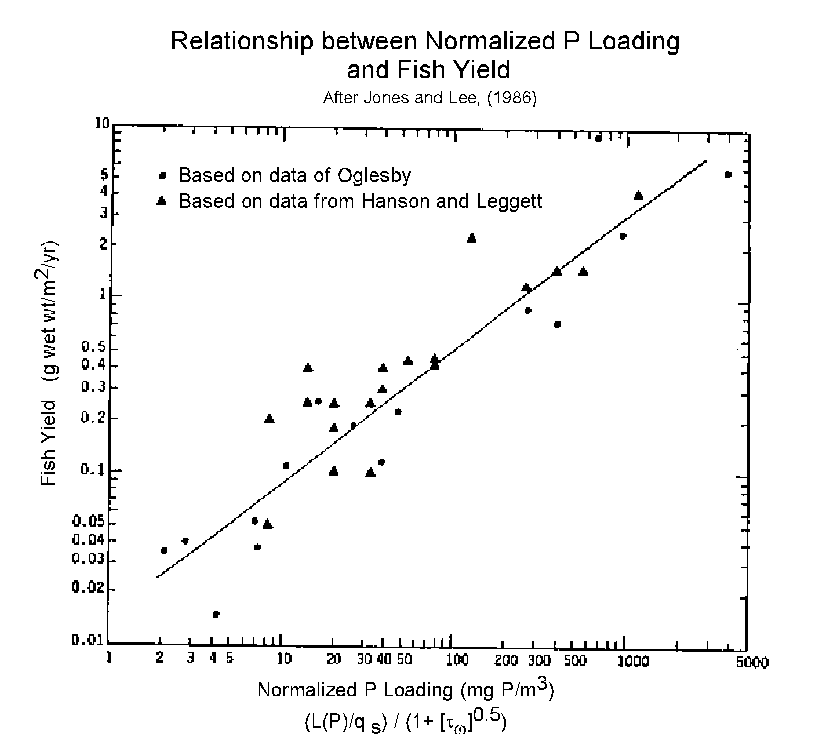
Literature Cited
Agee, B., Personal communication, April (1991).
Archibald, E. M., Personal communication, April (1991).
Archibald, E. M. and Lee, G. F., "Application of the OECD Eutrophication Modeling Approach to Lake Ray Hubbard, Texas," J. Am. Water Works Assoc. 73:590-599 (1981).
CH2M Hill, "Sanitary Landfill Database," (1989).
Cooke, G. D., Welch, E. B., Peterson, S. A., and Newroth, P. R., Lake and Reservoir Restoration, Butterworth Publishers, Stoneham, MA (1986).
DWR, "Sacramento-San Joaquin Delta Water Quality Surveillance Program 1985, Monitoring Results Pursuant to Delta Water Rights Decision 1485, Vol. 1," Dept. of Water Resources Report, Sacramento, CA, August (1986).
DWR, "The Delta as A Source of Drinking Water, Summary of Monitoring Results - 1983 to 1987," Interagency Delta Health Aspects Monitoring Program, Central District, Department of Water Resources, August (1989).
Folan, W. D., personal communication to G. Fred Lee, October (1989).
Gilbert, J. B., "Defining Water Quality in the 90's," Conference on Protecting Drinking Water Quality at the Source, Water Resources Center, University of California, Davis, CA, April (1991).
Glaze, W., "The Dilemma of Managing Disinfection By-products," Conference on Protecting Drinking Water Quality at the Source, Water Resources Center, University of California, Davis, CA, April (1991).
Goldman, C. R., "Primary Productivity, Nutrients, and Transparency during the Early Onset of Eutrophication in Ultra-oligotrophic Lake Tahoe, California-Nevada," Limnology and Oceanography 33:1321-1333 (1988).
Heyer, W. Personal communication. Supervising biologist with the Water Quality Division of the Los Angeles Water & Power, Los Angeles, California. March (1991).
Hoehn, R. C., Barnes, D. B., Randall, C. W., Grizzard, T. J., and Shaffer, T. B., "Algae as Sources of Trihalomethane Precursors," J. Am. Water Works Assoc. 72:344-350 (1980).
Jones, R. A. and Lee, G. F., "Chlorophyll-A Raw Water Quality Parameter," J. Am. Water Works Assoc. 74:490-494 (1982).
Jones, R. A. and Lee, G. F., "Eutrophication Modeling for Water Quality Management: An Update of the Vollenweider-OECD Model," World Health Organization's Water Quality Bulletin 11(2):67-74,118 (1986).
Jones, R. A. and Lee, G. F., "Evaluation of the Impact of Urbanization of a Forested Watershed on Eutrophication-Related Water Quality," Poster Presentation "California Watersheds at the Urban Interface," Third Biennial Conference, organized by the Watershed Management Council and the University of California Water Resources Center, Ontario, CA, October (1990).
Karimi, A. A. and Singer, P. C., "Trihalomethane Formation in Open Reservoirs," Journ. Amer. Water Works Assoc., 83:84-88 (1991).
Lee, G. F., "Eutrophication," Transactions of the Northeast Fish and Wildlife Conference, pp 39-60 (1973).
Lee, G. F. and Harlin, C. C., "Effect of Intake Location on Water Quality," Ind. Water Eng. 2:36-40 (1965).
Lee, G. F. and Jones, R. A., "Determination of the Nutrient Limiting Maximum Algal Biomass in Water Bodies," AWWA Quality Control in Reservoirs Committee report, NJIT Occasional Paper Department of Civil and Environmental Engineering, Newark, NJ, February (1981).
Lee, G. F. and Jones, R. A., "Managing Excessive Growths of Freshwater Sponge in the Canadian River Municipal Water Authority Raw Water Transmission Lines Without Excessive THM Formation," report to the Canadian River Municipal Water Authority by G. Fred Lee & Associates, Lubbock, TX (1983).
Lee, G. F. and Jones, R. A., "Predicting Domestic Water Supply Raw Water Quality in Proposed Impoundments," In: Proc. American Water Works Association 1984 Annual Conference Proceedings, pp 1611-1630 (1984a).
Lee, G. F. and Jones, R. A., "Is Hazardous Waste Disposal in Clay Vaults Safe?," J. American Water Works Association 76:66-73 (1984b).
Lee, G. F. and Jones, R. A., "Evaluation of Detergent Phosphate Bans on Water Quality," Lake Line, North American Lake Management Society 6:8-11 (1986).
Lee, G. F. and Jones, R. A., "The North American Experience in Eutrophication Control Through Phosphorus Management," Proc. Int. Conf. Phosphate, Water and Quality of Life, Paris, France, February (1988a).
Lee, G. F. and Jones, R. A., "Study Program for Development of Information for the Use of Vollenweider-OECD Eutrophication Modeling in Water Quality Management," AWWA Quality Control and Reservoirs Committee Report, NJIT Occasional Paper Department of Civil and Environmental Engineering, Newark, NJ, February (1988b).
Lee, G. F. and Jones, R. A., "Comments on Draft Statewide Water Quality Control Plans for: 1) Inland Surface Waters of California (California Inland Surface Waters Plan); 2) Enclosed Bays and Estuaries of California (California Enclosed Bays and Estuaries Plan) Dated November 2, 1990 and Functional Equivalent Document Dated November 26, 1990," submitted to the California Water Resources Control Board, Sacramento, CA, December (1990).
Lee, G. F. and Jones, R. A., "Municipal Solid Waste Management: Long-Term Public Health and Environmental Protection," Landfills and Groundwater Quality Short Course, University Extension, University of California, Davis, February (1991).
Lee, G. F., Jones, R. A., and Rast, W., "Availability of Phosphorus to Phytoplankton and Its Implication for Phosphorus Management Strategies," In: Phosphorus Management Strategies for Lakes, Ann Arbor Press, Ann Arbor, MI, pp 259-308 (1980).
Lee., G. F., Jones, R. A., and Ray, C., "Sanitary Landfill Leachate Recycle," BioCycle 27:36-38 (1986).
Lee, G. F., Rast, W., and Jones, R. A., "Eutrophication of Waterbodies: Insights for an Age-Old Problem," Environ. Sci. & Technol. 12:900-908 (1978).
Means, E., personal communication, Metropolitan Water District of Southern California, March (1991).
Palmer, C. M., "Algae in Water Supplies," US Public Health Service Publication No. 657, Washington, D.C. (1959).
Randtke, S. J., deNoyelles, F., Burkhead, C. E., Miller, R. E., Denne, J. E., Hathaway, L. R., and Melia, A. S., "Trihalomethane Precursors in Kansas Water Supplies: Occurrence, Source Control Measures, and Impacts on Drinking Water Treatment," Proc 38 Annual Environmental Engineering Conference, University of Kansas, Lawrence, KS (1988).
Rast, W. and Lee, G. F., "Summary Analysis of the North American (US Portion) OECD Eutrophication Project: Nutrient Loading-Lake Response Relationships and Trophic State Indices," EPA 600/3-78-008, US EPA-Corvallis (1978).
Rast, W. and Lee, G. F., "Nutrient Loading Estimates for Lakes," J. Environ. Engr. Div. ASCE 109:502-517 (1983). See also closure discussion, "Nutrient Estimates for Lakes," Journ. Environ. Engrg. 110:722-724 (1984).
Rast, W., Jones, R. A., and Lee, G. F., "Predictive Capability of US OECD Phosphorus Loading-Eutrophication Response Models," Journ. Water Pollut. Control Fed. 55:990-1003 (1983).
US EPA, "Draft Regulatory Impact Analysis of Proposed Revisions to Subtitle D Criteria for Municipal Solid Waste Landfills," prepared for the Economic Analysis Staff, Office of Solid Waste, Washington, DC, August (1988).
Walker, W. W., Jr., "Cause-Effect Relationships in the Eutrophication of Surface Water Supplies: Trihalomethanes," Conference on Water Quality and the Public Health, Worcester Department of Public Health, Concord, MA (1983).
Woodard, R. P., "Sources of Disinfection By-product Precursors," Conference on Protecting Drinking Water Quality at the Source, Water Resources Center, University of California, Davis, CA, April (1991).
WRCB, "Water Quality Plan for Salinity: San Francisco Bay/Sacramento-San Joaquin Delta Estuary," State of California State Water Resources Control Board, Sacramento, June (1990).
Reference as:"Lee, G. F. and Jones, R. A., "Regulating Drinking Water Quality at the Source," Proc. University of California Water Resources Center Conference: Protecting Water Supply Water Quality at the Source, Sacramento, CA, 39pp, April (1991)"

|
|